Pulmonary vascular inflammation with fatal coronavirus disease 2019 (COVID-19): possible role for the NLRP3 inflammasome
- PMID: 35144622
- PMCID: PMC8830114
- DOI: 10.1186/s12931-022-01944-8
Pulmonary vascular inflammation with fatal coronavirus disease 2019 (COVID-19): possible role for the NLRP3 inflammasome
Abstract
Background: Pulmonary hyperinflammation is a key event with SARS-CoV-2 infection. Acute respiratory distress syndrome (ARDS) that often accompanies COVID-19 appears to have worse outcomes than ARDS from other causes. To date, numerous lung histological studies in cases of COVID-19 have shown extensive inflammation and injury, but the extent to which these are a COVID-19 specific, or are an ARDS and/or mechanical ventilation (MV) related phenomenon is not clear. Furthermore, while lung hyperinflammation with ARDS (COVID-19 or from other causes) has been well studied, there is scarce documentation of vascular inflammation in COVID-19 lungs.
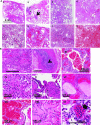
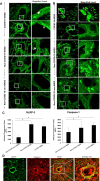
Methods: Lung sections from 8 COVID-19 affected and 11 non-COVID-19 subjects, of which 8 were acute respiratory disease syndrome (ARDS) affected (non-COVID-19 ARDS) and 3 were from subjects with non-respiratory diseases (non-COVID-19 non-ARDS) were H&E stained to ascertain histopathological features. Inflammation along the vessel wall was also monitored by expression of NLRP3 and caspase 1.
Results: In lungs from COVID-19 affected subjects, vascular changes in the form of microthrombi in small vessels, arterial thrombosis, and organization were extensive as compared to lungs from non-COVID-19 (i.e., non-COVID-19 ARDS and non-COVID-19 non-ARDS) affected subjects. The expression of NLRP3 pathway components was higher in lungs from COVID-19 ARDS subjects as compared to non-COVID-19 non-ARDS cases. No differences were observed between COVID-19 ARDS and non-COVID-19 ARDS lungs.
Conclusion: Vascular changes as well as NLRP3 inflammasome pathway activation were not different between COVID-19 and non-COVID-19 ARDS suggesting that these responses are not a COVID-19 specific phenomenon and are possibly more related to respiratory distress and associated strategies (such as MV) for treatment.
Keywords: COVID-19; Lung inflammation; Mechanical ventilation; Microthrombosis; NLRP3 inflammasome; Vascular endothelium.
© 2022. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures



Update of
-
Vascular Inflammation in Lungs of Patients with Fatal Coronavirus Disease 2019 (COVID-19) Infection: Possible role for the NLRP3 inflammasome.medRxiv [Preprint]. 2021 Mar 22:2021.03.19.21253815. doi: 10.1101/2021.03.19.21253815. medRxiv. 2021. Update in: Respir Res. 2022 Feb 10;23(1):25. doi: 10.1186/s12931-022-01944-8. PMID: 33791735 Free PMC article. Updated. Preprint.
-
Vascular Inflammation in Lungs of Patients with Fatal Coronavirus Disease 2019 (COVID-19): Possible Role for the NLRP3 Inflammasome.Res Sq [Preprint]. 2021 Sep 1:rs.3.rs-842167. doi: 10.21203/rs.3.rs-842167/v1. Res Sq. 2021. Update in: Respir Res. 2022 Feb 10;23(1):25. doi: 10.1186/s12931-022-01944-8. PMID: 34494018 Free PMC article. Updated. Preprint.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

