Postoperative delirium and changes in the blood-brain barrier, neuroinflammation, and cerebrospinal fluid lactate: a prospective cohort study
- PMID: 35144802
- PMCID: PMC9465948
- DOI: 10.1016/j.bja.2022.01.005
Postoperative delirium and changes in the blood-brain barrier, neuroinflammation, and cerebrospinal fluid lactate: a prospective cohort study
Abstract
Background: Case-control studies have associated delirium with blood-brain barrier (BBB) permeability. However, this approach cannot determine whether delirium is attributable to high pre-existing permeability or to perioperative changes. We tested whether perioperative changes in cerebrospinal fluid/plasma albumin ratio (CPAR) and plasma S100B were associated with delirium severity.
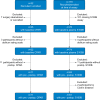
Methods: Participants were recruited to two prospective cohort studies of non-intracranial surgery (NCT01980511, NCT03124303, and NCT02926417). Delirium severity was assessed using the Delirium Rating Scale-98. Delirium incidence was diagnosed with the 3D-Confusion Assessment Method (3D-CAM) or CAM-ICU (CAM for the ICU). CSF samples from 25 patients and plasma from 78 patients were analysed for albumin and S100B. We tested associations between change in CPAR (n=11) and S100B (n=61) and delirium, blood loss, CSF interleukin-6 (IL-6), and CSF lactate.
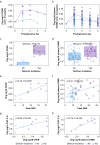
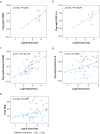
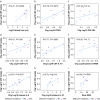
Results: The perioperative increase in CPAR and S100B correlated with delirium severity (CPAR ρ=0.78, P=0.01; S100B ρ=0.41, P<0.001), delirium incidence (CPAR P=0.012; S100B P<0.001) and CSF IL-6 (CPAR ρ=0.66 P=0.04; S100B ρ=0.75, P=0.025). Linear mixed-effect analysis also showed that decreased levels of S100B predicted recovery from delirium symptoms (P=0.001). Linear regression demonstrated that change in plasma S100B was independently associated with surgical risk, cardiovascular surgery, blood loss, and hypotension. Blood loss also correlated with CPAR (ρ=0.64, P=0.04), S100B (ρ=0.70, P<0.001), CSF lactate (R=0.81, P=0.01), and peak delirium severity (ρ=0.36, P=0.01).
Conclusion: Postoperative delirium is associated with a breakdown in the BBB. This increased permeability is dynamic and associated with a neuroinflammatory and lactate response. Strategies to mitigate blood loss may protect the BBB.
Keywords: delirium; dementia; inflammation; neuronal injury; older adults; surgery.
Copyright © 2022 British Journal of Anaesthesia. Published by Elsevier Ltd. All rights reserved.
Figures
Comment in
-
Breaking barriers in postoperative delirium.Br J Anaesth. 2022 Aug;129(2):147-150. doi: 10.1016/j.bja.2022.05.004. Epub 2022 Jun 17. Br J Anaesth. 2022. PMID: 35718561
References
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

