Phase II randomised trial of type I interferon inhibitor anifrolumab in patients with active lupus nephritis
- PMID: 35144924
- PMCID: PMC8921596
- DOI: 10.1136/annrheumdis-2021-221478
Phase II randomised trial of type I interferon inhibitor anifrolumab in patients with active lupus nephritis
Abstract
Objective: To assess the efficacy and safety of the type I interferon receptor antibody, anifrolumab, in patients with active, biopsy-proven, Class III/IV lupus nephritis.
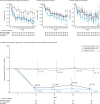
Methods: This phase II double-blinded study randomised 147 patients (1:1:1) to receive monthly intravenous anifrolumab basic regimen (BR, 300 mg), intensified regimen (IR, 900 mg ×3, 300 mg thereafter) or placebo, alongside standard therapy (oral glucocorticoids, mycophenolate mofetil). The primary endpoint was change in baseline 24-hour urine protein-creatinine ratio (UPCR) at week (W) 52 for combined anifrolumab versus placebo groups. The secondary endpoint was complete renal response (CRR) at W52. Exploratory endpoints included more stringent CRR definitions and sustained glucocorticoid reductions (≤7.5 mg/day, W24-52). Safety was analysed descriptively.
Results: Patients received anifrolumab BR (n=45), IR (n=51), or placebo (n=49). At W52, 24-hour UPCR improved by 69% and 70% for combined anifrolumab and placebo groups, respectively (geometric mean ratio=1.03; 95% CI 0.62 to 1.71; p=0.905). Serum concentrations were higher with anifrolumab IR versus anifrolumab BR, which provided suboptimal exposure. Numerically more patients treated with anifrolumab IR vs placebo attained CRR (45.5% vs 31.1%), CRR with UPCR ≤0.5 mg/mg (40.9% vs 26.7%), CRR with inactive urinary sediment (40.9% vs 13.3%) and sustained glucocorticoid reductions (55.6% vs 33.3%). Incidence of herpes zoster was higher with combined anifrolumab vs placebo (16.7% vs 8.2%). Incidence of serious adverse events was similar across groups.
Conclusion: Although the primary endpoint was not met, anifrolumab IR was associated with numerical improvements over placebo across endpoints, including CRR, in patients with active lupus nephritis.
Trial registration number: NCT02547922.
Keywords: autoimmune diseases; biological therapy; immune system diseases; lupus erythematosus; lupus nephritis; systemic.
© Author(s) (or their employer(s)) 2022. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: DJ received grants or contracts from GlaxoSmithKline, consultancy fees from AstraZeneca, Boehringer-Ingelheim, Chemocentryx, GlaxoSmithKline, Novartis, Roche, Takeda and Vifor, speaker fees from Amgen, GlaxoSmithKline and Vifor, and owns stocks in Aurinia. BR received consulting fees from Aurinia, AstraZeneca, Calliditas, Tavere, Novartis, Omeros, Chemocentryx, Morphosys, Bristol Myers Squibb, and Janssen. EM received consulting fees from Pfizer, AbbVie, GlaxoSmithKline, Sandoz, Eli Lilly, Bristol Myers Squibb and AstraZeneca, speaking fees from Pfizer, Amgen, AbbVie, Eli Lilly and Roche, and honoraria from Pfizer, AbbVie and Eli Lilly. RAF received consulting fees, payment or honoraria, and support for attending meetings and/or travel from AstraZeneca, and has participated on a Data Safety Monitoring Board or Advisory Board for AstraZeneca. FAH has received grants and consulting fees from and has participated on a Data Safety Monitoring Board or Advisory Board for GlaxoSmithKline, and has received consulting fees from Idorsia. TT, JK, ES, RT and CL are employees of and own shares of AstraZeneca. YLC is a former employee of and owns shares of AstraZeneca and a current employee of Seagen.
Figures


Comment in
-
Interferon blockade in lupus: effects on antiviral immunity.Nat Rev Nephrol. 2022 Jul;18(7):415-416. doi: 10.1038/s41581-022-00581-0. Nat Rev Nephrol. 2022. PMID: 35523957 Free PMC article.
References
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

