Effectiveness of mRNA vaccines and waning of protection against SARS-CoV-2 infection and severe covid-19 during predominant circulation of the delta variant in Italy: retrospective cohort study
- PMID: 35144968
- PMCID: PMC8829820
- DOI: 10.1136/bmj-2021-069052
Effectiveness of mRNA vaccines and waning of protection against SARS-CoV-2 infection and severe covid-19 during predominant circulation of the delta variant in Italy: retrospective cohort study
Abstract
Objectives: To estimate the effectiveness of mRNA vaccines against SARS-CoV-2 infection and severe covid-19 at different time after vaccination.
Design: Retrospective cohort study.
Setting: Italy, 27 December 2020 to 7 November 2021.
Participants: 33 250 344 people aged ≥16 years who received a first dose of BNT162b2 (Pfizer-BioNTech) or mRNA-1273 (Moderna) vaccine and did not have a previous diagnosis of SARS-CoV-2 infection.
Main outcome measures: SARS-CoV-2 infection and severe covid-19 (admission to hospital or death). Data were divided by weekly time intervals after vaccination. Incidence rate ratios at different time intervals were estimated by multilevel negative binomial models with robust variance estimator. Sex, age group, brand of vaccine, priority risk category, and regional weekly incidence in the general population were included as covariates. Geographic region was included as a random effect. Adjusted vaccine effectiveness was calculated as (1-IRR)×100, where IRR=incidence rate ratio, with the time interval 0-14 days after the first dose of vaccine as the reference.
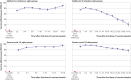
Results: During the epidemic phase when the delta variant was the predominant strain of the SARS-CoV-2 virus, vaccine effectiveness against SARS-CoV-2 infection significantly decreased (P<0.001) from 82% (95% confidence interval 80% to 84%) at 3-4 weeks after the second dose of vaccine to 33% (27% to 39%) at 27-30 weeks after the second dose. In the same time intervals, vaccine effectiveness against severe covid-19 also decreased (P<0.001), although to a lesser extent, from 96% (95% to 97%) to 80% (76% to 83%). High risk people (vaccine effectiveness -6%, -28% to 12%), those aged ≥80 years (11%, -15% to 31%), and those aged 60-79 years (2%, -11% to 14%) did not seem to be protected against infection at 27-30 weeks after the second dose of vaccine.
Conclusions: The results support the vaccination campaigns targeting high risk people, those aged ≥60 years, and healthcare workers to receive a booster dose of vaccine six months after the primary vaccination cycle. The results also suggest that timing the booster dose earlier than six months after the primary vaccination cycle and extending the offer of the booster dose to the wider eligible population might be warranted.
© Author(s) (or their employer(s)) 2019. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: All authors have completed the ICMJE uniform disclosure form at www.icmje.org/disclosure-of-interest/ and declare: no support from any organisation for the submitted work; no financial relationships with any organisations that might have an interest in the submitted work in the previous three years; no other relationships or activities that could appear to have influenced the submitted work.
Figures



References
-
- Italian Government, Presidency of the Council of Ministers. Data repository. https://www.governo.it/it/dipartimenti/commissario-straordinario-lemerge...
-
- Haas EJ, Angulo FJ, McLaughlin JM, et al. . Impact and effectiveness of mRNA BNT162b2 vaccine against SARS-CoV-2 infections and COVID-19 cases, hospitalisations, and deaths following a nationwide vaccination campaign in Israel: an observational study using national surveillance data. Lancet 2021;397:1819-29. 10.1016/S0140-6736(21)00947-8 - DOI - PMC - PubMed
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
