Seroresponse to SARS-CoV-2 Vaccines among Maintenance Dialysis Patients over 6 Months
- PMID: 35144972
- PMCID: PMC8975038
- DOI: 10.2215/CJN.12250921
Seroresponse to SARS-CoV-2 Vaccines among Maintenance Dialysis Patients over 6 Months
Abstract
Background and objectives: Although most patients receiving maintenance dialysis exhibit initial seroresponse to severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) vaccination, concerns exist regarding the durability of this antibody response. This study evaluated seroresponse over time.
Design, setting, participants, & measurements: This retrospective cohort study included patients on maintenance dialysis, from a midsize national dialysis provider, who received a complete SARS-CoV-2 vaccine series and had at least one antibody titer checked after full vaccination. IgG spike antibodies (anti-spike IgG) titers were assessed monthly with routine laboratory tests after vaccination; the semiquantitative assay reported a range between zero and ≥20 Index. Descriptive analyses compared trends over time by history of coronavirus disease 2019 (COVID-19) and vaccine type. Time-to-event analyses examined the outcome of loss of seroresponse (anti-spike IgG <1 Index or development of COVID-19). Cox regression adjusted for additional clinical characteristics.
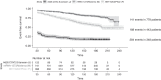
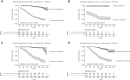
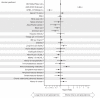
Results: Among 1870 patients receiving maintenance dialysis, 1569 had no prior COVID-19. Patients without prior COVID-19 had declining titers over time. Among 443 recipients of BNT162b2 (Pfizer), median (interquartile range) anti-spike IgG titer declined from ≥20 (5.89 to ≥20) in month 1 after full vaccination to 1.96 (0.60-5.88) by month 6. Among 778 recipients of mRNA-1273 (Moderna), anti-spike IgG titer declined from ≥20 (interquartile range, ≥20 to ≥20) in month 1 to 7.99 (2.61 to ≥20) by month 6. The 348 recipients of Ad26.COV2.S (Janssen) had a lower titer response than recipients of an mRNA vaccine over all time periods. In time-to-event analyses, recipients of Ad26.COV2.S and mRNA-1273 had the shortest and longest time to loss of seroresponse, respectively. The maximum titer reached in the first 2 months after full vaccination was associated with durability of the anti-spike IgG seroresponse; patients with anti-spike IgG titer 1-19.99 had a shorter time to loss of seroresponse compared with patients with anti-spike IgG titer ≥20 (hazard ratio, 15.5; 95% confidence interval, 11.7 to 20.7).
Conclusions: Among patients receiving maintenance dialysis, vaccine-induced seroresponse wanes over time across vaccine types. Early titers after full vaccination are associated with the durability of seroresponse.
Keywords: COVID-19; COVID-19 vaccines; SARS-CoV-2; chronic hemodialysis; end-stage renal disease; maintenance; peritoneal dialysis.
Copyright © 2022 by the American Society of Nephrology.
Figures



Comment in
- 335–337 doi: 10.2215/CJN.00320122
References
-
- World Health Organization : WHO coronavirus disease (COVID-19) dashboard, 2021. Available at: https://covid19.who.int/. Accessed September 6, 2021
-
- Polack FP, Thomas SJ, Kitchin N, Absalon J, Gurtman A, Lockhart S, Perez JL, Pérez Marc G, Moreira ED, Zerbini C, Bailey R, Swanson KA, Roychoudhury S, Koury K, Li P, Kalina WV, Cooper D, Frenck RW Jr, Hammitt LL, Türeci Ö, Nell H, Schaefer A, Ünal S, Tresnan DB, Mather S, Dormitzer PR, Şahin U, Jansen KU, Gruber WC; C4591001 Clinical Trial Group : Safety and efficacy of the BNT162b2 mRNA covid-19 vaccine. N Engl J Med 383: 2603–2615, 2020 - PMC - PubMed
-
- Baden LR, El Sahly HM, Essink B, Kotloff K, Frey S, Novak R, Diemert D, Spector SA, Rouphael N, Creech CB, McGettigan J, Khetan S, Segall N, Solis J, Brosz A, Fierro C, Schwartz H, Neuzil K, Corey L, Gilbert P, Janes H, Follmann D, Marovich M, Mascola J, Polakowski L, Ledgerwood J, Graham BS, Bennett H, Pajon R, Knightly C, Leav B, Deng W, Zhou H, Han S, Ivarsson M, Miller J, Zaks T; COVE Study Group : Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med 384: 403–416, 2021 - PMC - PubMed
-
- Sadoff J, Gray G, Vandebosch A, Cárdenas V, Shukarev G, Grinsztejn B, Goepfert PA, Truyers C, Fennema H, Spiessens B, Offergeld K, Scheper G, Taylor KL, Robb ML, Treanor J, Barouch DH, Stoddard J, Ryser MF, Marovich MA, Neuzil KM, Corey L, Cauwenberghs N, Tanner T, Hardt K, Ruiz-Guiñazú J, Le Gars M, Schuitemaker H, Van Hoof J, Struyf F, Douoguih M; ENSEMBLE Study Group : Safety and efficacy of single-dose Ad26.COV2.S vaccine against Covid-19. N Engl J Med 384: 2187–2201, 2021 - PMC - PubMed
-
- Brosh-Nissimov T, Orenbuch-Harroch E, Chowers M, Elbaz M, Nesher L, Stein M, Maor Y, Cohen R, Hussein K, Weinberger M, Zimhony O, Chazan B, Najjar R, Zayyad H, Rahav G, Wiener-Well Y: BNT162b2 vaccine breakthrough: Clinical characteristics of 152 fully vaccinated hospitalized COVID-19 patients in Israel. Clin Microbiol Infect 27: 1652–1657, 2021 - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

