Associative detachment in anion-atom reactions involving a dipole-bound electron
- PMID: 35145072
- PMCID: PMC8831523
- DOI: 10.1038/s41467-022-28382-w
Associative detachment in anion-atom reactions involving a dipole-bound electron
Abstract
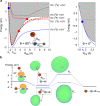
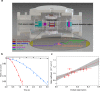
Associative electronic detachment (AED) between anions and neutral atoms leads to the detachment of the anion's electron resulting in the formation of a neutral molecule. It plays a key role in chemical reaction networks, like the interstellar medium, the Earth's ionosphere and biochemical processes. Here, a class of AED involving a closed-shell anion (OH-) and alkali atoms (rubidium) is investigated by precisely controlling the fraction of electronically excited rubidium. Reaction with the ground state atom gives rise to a stable intermediate complex with an electron solely bound via dipolar forces. The stability of the complex is governed by the subtle interplay of diabatic and adiabatic couplings into the autodetachment manifold. The measured rate coefficients are in good agreement with ab initio calculations, revealing pronounced steric effects. For excited state rubidium, however, a lower reaction rate is observed, indicating dynamical stabilization processes suppressing the coupling into the autodetachment region. Our work provides a stringent test of ab initio calculations on anion-neutral collisions and constitutes a generic, conceptual framework for understanding electronic state dependent dynamics in AEDs.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures
References
-
- Lambert, J. L. Trace inorganics in water, vol. 73 of Adv. Chem. Ser., chap. 2, 18–26 (American Chemical Society, 1968).
-
- Fehsenfeld FC, Albritton DL, Burt JA, Schiff HI. Associative-detachment reactions of O− and O by O2(1Δg) Can. J. Chem. 1969;47:1793–1795.
-
- Bierbaum VM. Anions in space and in the laboratory. Proc. Int. Astron. Union. 2011;7:383–389.
-
- Herbst E. Can negative molecular ions be detected in dense interstellar clouds? Nature. 1981;289:656–657.
-
- Millar TJ, Walsh C, Field TA. Negative ions in space. Chem. Rev. 2017;117:1765–1795. - PubMed
Grants and funding
- WE2661/14-1/Deutsche Forschungsgemeinschaft (German Research Foundation)
- WE2661/14-1/Deutsche Forschungsgemeinschaft (German Research Foundation)
- WE2661/14-1/Deutsche Forschungsgemeinschaft (German Research Foundation)
- WE2661/14-1/Deutsche Forschungsgemeinschaft (German Research Foundation)
- MeSoX 05K19GUE/Bundesministerium für Bildung und Forschung (Federal Ministry of Education and Research)
LinkOut - more resources
Full Text Sources

