Connexin 43: insights into candidate pathological mechanisms of depression and its implications in antidepressant therapy
- PMID: 35145238
- PMCID: PMC9525669
- DOI: 10.1038/s41401-022-00861-2
Connexin 43: insights into candidate pathological mechanisms of depression and its implications in antidepressant therapy
Abstract
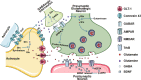
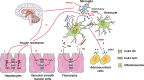
Major depressive disorder (MDD), a chronic and recurrent disease characterized by anhedonia, pessimism or even suicidal thought, remains a major chronic mental concern worldwide. Connexin 43 (Cx43) is the most abundant connexin expressed in astrocytes and forms the gap junction channels (GJCs) between astrocytes, the most abundant and functional glial cells in the brain. Astrocytes regulate neurons' synaptic strength and function by expressing receptors and regulating various neurotransmitters. Astrocyte dysfunction causes synaptic abnormalities, which are related to various mood disorders, e.g., depression. Increasing evidence suggests a crucial role of Cx43 in the pathogenesis of depression. Depression down-regulates Cx43 expression in humans and rats, and dysfunction of Cx43 also induces depressive behaviors in rats and mice. Recently Cx43 has received considerable critical attention and is highly implicated in the onset of depression. However, the pathological mechanisms of depression-like behavior associated with Cx43 still remain ambiguous. In this review we summarize the recent progress regarding the underlying mechanisms of Cx43 in the etiology of depression-like behaviors including gliotransmission, metabolic disorders, and neuroinflammation. We also discuss the effects of antidepressants (monoamine antidepressants and ketamine) on Cx43. The clarity of the candidate pathological mechanisms of depression-like behaviors associated with Cx43 and its potential pharmacological roles for antidepressants will benefit the exploration of a novel antidepressant target.
Keywords: ATP; Ca2+ wave; Connexin 43; antidepressant target; astrocyte; depression; hippocampus; prefrontal cortex.
© 2022. The Author(s), under exclusive licence to Shanghai Institute of Materia Medica, Chinese Academy of Sciences and Chinese Pharmacological Society.
Conflict of interest statement
The authors declare no competing interests.
Figures


Similar articles
-
Connexin 43: An Interface Connecting Neuroinflammation to Depression.Molecules. 2023 Feb 15;28(4):1820. doi: 10.3390/molecules28041820. Molecules. 2023. PMID: 36838809 Free PMC article. Review.
-
Astroglial Connexin 43-Mediated Gap Junctions and Hemichannels: Potential Antidepressant Mechanisms and the Link to Neuroinflammation.Cell Mol Neurobiol. 2023 Nov;43(8):4023-4040. doi: 10.1007/s10571-023-01426-5. Epub 2023 Oct 24. Cell Mol Neurobiol. 2023. PMID: 37875763 Free PMC article. Review.
-
Amelioration of gap junction dysfunction in a depression model by loganin: Involvement of GSK-3β/β-catenin signaling.J Ethnopharmacol. 2025 Feb 11;341:119288. doi: 10.1016/j.jep.2024.119288. Epub 2024 Dec 26. J Ethnopharmacol. 2025. PMID: 39732296
-
Gap junction channels as potential targets for the treatment of major depressive disorder.Psychopharmacology (Berl). 2018 Jan;235(1):1-12. doi: 10.1007/s00213-017-4782-7. Epub 2017 Nov 25. Psychopharmacology (Berl). 2018. PMID: 29178009 Review.
-
Dysfunction of astrocytic connexins 30 and 43 in the medial prefrontal cortex and hippocampus mediates depressive-like behaviours.Behav Brain Res. 2019 Oct 17;372:111950. doi: 10.1016/j.bbr.2019.111950. Epub 2019 May 16. Behav Brain Res. 2019. PMID: 31103752
Cited by
-
Connexin 43: An Interface Connecting Neuroinflammation to Depression.Molecules. 2023 Feb 15;28(4):1820. doi: 10.3390/molecules28041820. Molecules. 2023. PMID: 36838809 Free PMC article. Review.
-
Perspective and Therapeutic Potential of the Noncoding RNA-Connexin Axis.Int J Mol Sci. 2024 Jun 2;25(11):6146. doi: 10.3390/ijms25116146. Int J Mol Sci. 2024. PMID: 38892334 Free PMC article. Review.
-
Astroglial Connexin 43-Mediated Gap Junctions and Hemichannels: Potential Antidepressant Mechanisms and the Link to Neuroinflammation.Cell Mol Neurobiol. 2023 Nov;43(8):4023-4040. doi: 10.1007/s10571-023-01426-5. Epub 2023 Oct 24. Cell Mol Neurobiol. 2023. PMID: 37875763 Free PMC article. Review.
-
The neurobiological mechanisms and therapeutic prospect of extracellular ATP in depression.CNS Neurosci Ther. 2024 Feb;30(2):e14536. doi: 10.1111/cns.14536. CNS Neurosci Ther. 2024. PMID: 38375982 Free PMC article. Review.
-
Adaptogens on Depression-Related Outcomes: A Systematic Integrative Review and Rationale of Synergism with Physical Activity.Int J Environ Res Public Health. 2023 Mar 28;20(7):5298. doi: 10.3390/ijerph20075298. Int J Environ Res Public Health. 2023. PMID: 37047914 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

