Continous, non-invasive monitoring of oxygen consumption in a parallelized microfluidic in vitro system provides novel insight into the response to nutrients and drugs of primary human hepatocytes
- PMID: 35145369
- PMCID: PMC8822303
- DOI: 10.17179/excli2021-4351
Continous, non-invasive monitoring of oxygen consumption in a parallelized microfluidic in vitro system provides novel insight into the response to nutrients and drugs of primary human hepatocytes
Abstract
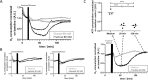
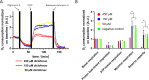
Oxygen plays a fundamental role in cellular energy metabolism, differentiation and cell biology in general. Consequently, in vitro oxygen sensing can be used to assess cell vitality and detect specific mechanisms of toxicity. In 2D in vitro models currently used, the oxygen supply provided by diffusion is generally too low, especially for cells having a high oxygen demand. In organ-on-chip systems, a more physiologic oxygen supply can be generated by establishing unidirectional perfusion. We established oxygen sensors in an easy-to-use and parallelized organ-on-chip system. We demonstrated the applicability of this system by analyzing the influence of fructose (40 mM, 80 mM), ammonium chloride (100 mM) and Na-diclofenac (50 µM, 150 µM, 450 µM, 1500 µM) on primary human hepatocytes (PHH). Fructose treatment for two hours showed an immediate drop of oxygen consumption (OC) with subsequent increase to nearly initial levels. Treatment with 80 mM glucose, 20 mM lactate or 20 mM glycerol did not result in any changes in OC which demonstrates a specific effect of fructose. Application of ammonium chloride for two hours did not show any immediate effects on OC, but qualitatively changed the cellular response to FCCP treatment. Na-diclofenac treatment for 24 hours led to a decrease of the maximal respiration and reserve capacity. We also demonstrated the stability of our system by repeatedly treating cells with 40 mM fructose, which led to similar cell responses on the same day as well as on subsequent days. In conclusion, our system enables in depth analysis of cellular respiration after substrate treatment in an unidirectional perfused organ-on-chip system.
Keywords: in vitro; liver; metabolism; model; organ-on-chip; oxygen; perfusion; sensors; toxicity.
Copyright © 2022 Busche et al.
Figures




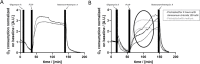
Similar articles
-
Online oxygen monitoring using integrated inkjet-printed sensors in a liver-on-a-chip system.Lab Chip. 2018 Jul 10;18(14):2023-2035. doi: 10.1039/c8lc00456k. Lab Chip. 2018. PMID: 29892739
-
HepaChip-MP - a twenty-four chamber microplate for a continuously perfused liver coculture model.Lab Chip. 2020 Aug 11;20(16):2911-2926. doi: 10.1039/d0lc00357c. Lab Chip. 2020. PMID: 32662810
-
An effect of extracellular redox state on the glucagon-stimulated glucose release by rat hepatocytes and perfused liver.Horm Metab Res. 1977 May;9(3):213-7. doi: 10.1055/s-0028-1093539. Horm Metab Res. 1977. PMID: 885474
-
New hepatocyte in vitro systems for drug metabolism: metabolic capacity and recommendations for application in basic research and drug development, standard operation procedures.Drug Metab Rev. 2003 May-Aug;35(2-3):145-213. doi: 10.1081/dmr-120023684. Drug Metab Rev. 2003. PMID: 12959414 Review.
-
Drug metabolism by cultured human hepatocytes: how far are we from the in vivo reality?Altern Lab Anim. 2004 Jun;32(2):101-10. doi: 10.1177/026119290403200207. Altern Lab Anim. 2004. PMID: 15601238 Review.
Cited by
-
On-chip analysis of glycolysis and mitochondrial respiration in human induced pluripotent stem cells.Mater Today Bio. 2022 Oct 31;17:100475. doi: 10.1016/j.mtbio.2022.100475. eCollection 2022 Dec 15. Mater Today Bio. 2022. PMID: 36388452 Free PMC article.
References
-
- Aduen J, Bernstein WK, Khastgir T, Miller J, Kerzner R, Bhatiani A, et al. The use and clinical importance of a substrate-specific electrode for rapid determination of blood lactate concentrations. JAMA. 1994;272:1678–1685. - PubMed
LinkOut - more resources
Full Text Sources
