A Putative Role for Ubiquitin-Proteasome Signaling in Estrogenic Memory Regulation
- PMID: 35145382
- PMCID: PMC8821141
- DOI: 10.3389/fnbeh.2021.807215
A Putative Role for Ubiquitin-Proteasome Signaling in Estrogenic Memory Regulation
Abstract
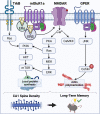
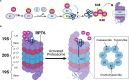
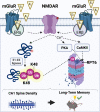
Sex steroid hormones such as 17β-estradiol (E2) are critical neuromodulators of hippocampal synaptic plasticity and hippocampus-dependent memory in both males and females. However, the mechanisms through which E2 regulates memory formation in both sexes remain unclear. Research to date suggests that E2 regulates hippocampus-dependent memory by activating numerous cell-signaling cascades to promote the synthesis of proteins that support structural changes at hippocampal synapses. However, this work has largely overlooked the equally important contributions of protein degradation mediated by the ubiquitin proteasome system (UPS) in remodeling the synapse. Despite being critically implicated in synaptic plasticity and successful formation of long-term memories, it remains unclear whether protein degradation mediated by the UPS is necessary for E2 to exert its beneficial effects on hippocampal plasticity and memory formation. The present article provides an overview of the receptor and signaling mechanisms so far identified as critical for regulating hippocampal E2 and UPS function in males and females, with a particular emphasis on the ways in which these mechanisms overlap to support structural integrity and protein composition of hippocampal synapses. We argue that the high degree of correspondence between E2 and UPS activity warrants additional study to examine the contributions of ubiquitin-mediated protein degradation in regulating the effects of sex steroid hormones on cognition.
Keywords: 17β-estradiol; hippocampus; memory; proteasome; protein degradation; ubiquitin.
Copyright © 2022 Beamish and Frick.
Conflict of interest statement
KF is a co-founder of, and shareholder in, Estrigenix Therapeutics, Inc., a company which aims to improve women’s health by developing safe, clinically proven treatment for the mental and physical effects of menopause. She also serves as the company’s Chief Scientific Officer. The remaining author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
Neuron-Derived Estrogen Regulates Synaptic Plasticity and Memory.J Neurosci. 2019 Apr 10;39(15):2792-2809. doi: 10.1523/JNEUROSCI.1970-18.2019. Epub 2019 Feb 6. J Neurosci. 2019. PMID: 30728170 Free PMC article.
-
The Ubiquitin-Proteasome System and Memory: Moving Beyond Protein Degradation.Neuroscientist. 2018 Dec;24(6):639-651. doi: 10.1177/1073858418762317. Epub 2018 Mar 12. Neuroscientist. 2018. PMID: 29529924 Review.
-
Estrogenic regulation of memory consolidation: A look beyond the hippocampus, ovaries, and females.Physiol Behav. 2018 Apr 1;187:57-66. doi: 10.1016/j.physbeh.2017.07.028. Epub 2017 Jul 27. Physiol Behav. 2018. PMID: 28755863 Free PMC article. Review.
-
The ubiquitin-proteasome system and learning-dependent synaptic plasticity - A 10 year update.Neurosci Biobehav Rev. 2023 Sep;152:105280. doi: 10.1016/j.neubiorev.2023.105280. Epub 2023 Jun 12. Neurosci Biobehav Rev. 2023. PMID: 37315660 Free PMC article. Review.
-
Intriguing roles of hippocampus-synthesized 17β-estradiol in the modulation of hippocampal synaptic plasticity.J Mol Neurosci. 2014;54(2):271-81. doi: 10.1007/s12031-014-0285-8. Epub 2014 Apr 15. J Mol Neurosci. 2014. PMID: 24729128 Review.
Cited by
-
Paternal preconceptional diet enriched with n-3 polyunsaturated fatty acids affects offspring brain function in mice.Front Nutr. 2022 Oct 28;9:969848. doi: 10.3389/fnut.2022.969848. eCollection 2022. Front Nutr. 2022. PMID: 36386900 Free PMC article.
-
Sex-differences in proteasome-dependent K48-polyubiquitin signaling in the amygdala are developmentally regulated in rats.Biol Sex Differ. 2023 Nov 10;14(1):80. doi: 10.1186/s13293-023-00566-z. Biol Sex Differ. 2023. PMID: 37950270 Free PMC article.
-
Proteasome-independent K63 polyubiquitination selectively regulates ATP levels and proteasome activity during fear memory formation in the female amygdala.Mol Psychiatry. 2023 Jun;28(6):2594-2605. doi: 10.1038/s41380-023-02112-0. Epub 2023 May 17. Mol Psychiatry. 2023. PMID: 37198264 Free PMC article.
-
Bioinformatics analysis of diagnostic biomarkers for Alzheimer's disease in peripheral blood based on sex differences and support vector machine algorithm.Hereditas. 2022 Oct 4;159(1):38. doi: 10.1186/s41065-022-00252-x. Hereditas. 2022. PMID: 36195955 Free PMC article.
-
The epigenetic role of proteasome subunit RPT6 during memory formation in female rats.Learn Mem. 2022 Sep 2;29(9):256-264. doi: 10.1101/lm.053498.121. Print 2022 Sep. Learn Mem. 2022. PMID: 36206393 Free PMC article.
References
-
- Artinian J., McGauran A., De Jaeger X., Mouledous L., Frances B., Roullet P., et al. . (2008). Protein degradation, as with protein synthesis, is required during not only long-term spatial memory consolidation but also reconsolidation. Eur. J. Neurosci. 27, 3009–3019. 10.1111/j.1460-9568.2008.06262.x - DOI - PubMed
LinkOut - more resources
Full Text Sources

