Prediction of the Effects of Empagliflozin on Cardiovascular and Kidney Outcomes Based on Short-Term Changes in Multiple Risk Markers
- PMID: 35145402
- PMCID: PMC8821652
- DOI: 10.3389/fphar.2021.786706
Prediction of the Effects of Empagliflozin on Cardiovascular and Kidney Outcomes Based on Short-Term Changes in Multiple Risk Markers
Abstract
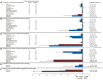
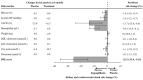
Aims: The EMPA-REG OUTCOME trial demonstrated that the sodium-glucose cotransporter-2 inhibitor (SGLT2) empagliflozin reduces the risk of cardiovascular (CV) and kidney outcomes in patients with type 2 diabetes. We previously developed the parameter response efficacy (PRE) score, which translates drug effects on multiple short-term risk markers into a predicted long-term treatment effect on clinical outcomes. The main objective of this study was to assess the accuracy of the PRE score in predicting the efficacy of empagliflozin in reducing the risk of CV and kidney outcomes. Methods: Short-term (baseline to 6-months) changes in glycated hemoglobin (HbA1c), systolic blood pressure (SBP), urinary-albumin-creatinine-ratio (UACR), hemoglobin, body weight, high-density-lipoprotein (HDL) cholesterol, low-density-lipoprotein (LDL) cholesterol, uric acid, and potassium were determined among 7020 patients with type 2 diabetes and established CV disease in the EMPA-REG OUTCOME trial. The beta-coefficients, derived from a Cox proportional hazards model in a pooled database consisting of 6355 patients with type 2 diabetes, were applied to the short-term risk markers in the EMPA-REG OUTCOME trial to predict the empagliflozin-induced impact on CV (defined as a composite of non-fatal myocardial infarction, non-fatal stroke, or CV death) and kidney (defined as a composite of doubling of serum creatinine or end-stage kidney disease) outcomes. Results: Empagliflozin compared to placebo reduced HbA1c (0.6%), SBP (4.2 mmHg), UACR (13.0%), body weight (2.1 kg), uric acid (20.4 μmol/L), and increased hemoglobin (6.6 g/L), LDL-cholesterol (0.1 mmol/L) and HDL-cholesterol (0.04 mmol/L) (all p<0.01). Integrating these effects in the PRE score resulted in a predicted relative risk reduction (RRR) for the CV outcome of 6.4% (95% CI 1.4-11.7), which was less than the observed 14.7% (95% CI 1.3-26.4%) RRR. For the kidney outcome, the PRE score predicted a RRR of 33.4% (95% CI 26.2-39.8); the observed RRR was 46.9% (95% CI 26.8-61.5). In a subgroup of 2,811 patients with UACR ≥30 mg/g at baseline, the PRE score predicted RRR was 40.8% (95% CI 31.2-49.1) vs. the observed RRR of 40.8% (95% CI 12.4-60.0) for the kidney outcome. Conclusions: Integrating multiple short-term risk marker changes in the PRE score underestimated the effect of empagliflozin on CV and kidney outcomes, suggesting that the currently used risk markers do not fully capture the effect of empagliflozin. In patients with increased albuminuria, the PRE score adequately predicted the effect of empagliflozin on kidney outcomes.
Keywords: cardiovascular outcomes; diabetes; empagliflozin; kidney outcomes; risk markers.
Copyright © 2022 Tye, de Vries, Wanner, Denig and Heerspink.
Conflict of interest statement
CW has received honoraria for consultancy and lecturing from AstraZeneca, Bayer, BI, GlaxoSmithKline, Eli Lilly and Company, Merck Sharp & Dome, Mundipharma, Sanofi Genzyme, and Takeda. HH is a consultant for AbbVie, Astellas, AstraZeneca, Boehringer Ingelheim, Fresenius, Gilead, Janssen, Merck, Mitsubishi Tanabe and MundiPharma and has a policy that all honoraria are paid to his employer. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures




References
-
- Anker S. D., Butler J., Filippatos G., Khan M. S., Marx N., Lam C. S. P., et al. (2021). Effect of Empagliflozin on Cardiovascular and Renal Outcomes in Patients with Heart Failure by Baseline Diabetes Status: Results from the EMPEROR-Reduced Trial. Circulation 143, 337–349. 10.1161/CIRCULATIONAHA.120.051824 - DOI - PMC - PubMed
-
- Barnett A. H., Mithal A., Manassie J., Jones R., Rattunde H., Woerle H. J., et al. (2014). Efficacy and Safety of Empagliflozin Added to Existing Antidiabetes Treatment in Patients with Type 2 Diabetes and Chronic Kidney Disease: a Randomised, Double-Blind, Placebo-Controlled Trial. Lancet Diabetes Endocrinol. 2, 369–384. 10.1016/S2213-8587(13)70208-0 - DOI - PubMed
LinkOut - more resources
Full Text Sources

