Methylxanthine Treatment in Patients Hospitalized for Acute Exacerbation of Chronic Obstructive Pulmonary Disease in China: A Real-World Study Using Propensity Score Matching Analysis
- PMID: 35145412
- PMCID: PMC8821534
- DOI: 10.3389/fphar.2022.802123
Methylxanthine Treatment in Patients Hospitalized for Acute Exacerbation of Chronic Obstructive Pulmonary Disease in China: A Real-World Study Using Propensity Score Matching Analysis
Abstract
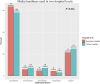
Background: Although medical guidelines discourage the use of methylxanthines in patients with acute exacerbation of chronic obstructive pulmonary disease (AECOPD), they are still widely used in clinical practice. This study investigated the real-world use of methylxanthines in the management of AECOPD. Methods: Patient data from the Acute exacerbation of Chronic obstructive pulmonary disease Using REgistry data (ACURE, NCT02657525) study database were screened. Enrolled patients were divided into treatment and control groups. Propensity score (PS) matching and Cox regression analyses were used to minimize confounding factors and determine the association between methylxanthine treatment and the length of stay (LOS). Results: Among the 2088 eligible patients, 1,563 (74.9%) were in the methylxanthine treatment group. Patients treated with methylxanthines had more severe respiratory symptoms and worse lung function than those in the control group. Doxophylline was the most commonly used methylxanthine in both secondary and tertiary hospitals. After PS matching, 966 patients were equally divided into two groups. The LOS of patients in the two groups was similar [median: 8 days, interquartile range (IQR): 7-11 days, p = 0.730]. Patients in the treatment group (median: 8, IQR: 4-12) had a more significant decrease in the COPD Assessment Test score from admission to discharge than those in the control group (median: 6, IQR: 2-10, p < 0.001). Among all matched patients, the LOS was not significantly associated with methylxanthine treatment [adjusted hazard ratio (HR): 1.02, 95% confidence intervals (CIs): 0.89-1.16]. However, in the subgroup analysis, methylxanthines were significantly associated with a short LOS in patients with blood eosinophil count >4% (adjusted HR: 1.56, 95% CIs: 1.12-2.17). Conclusion: This study revealed that methylxanthines, especially doxophylline, are widely used in China. Methylxanthines were effective in improving symptoms in AECOPD patients. Higher blood eosinophil count may be associated with a better efficacy of methylxanthine treatment.
Keywords: acute exacerbation; chronic obstructive pulmonary disease; hospitalization; length of stay; methylxanthine.
Copyright © 2022 Zhan, Ma, Huang, Liang, Mao, Zhang, Ren, Lei, Chen, Yang and Wang.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


Similar articles
-
Blood Eosinophils and Clinical Outcomes in Patients With Acute Exacerbation of Chronic Obstructive Pulmonary Disease: A Propensity Score Matching Analysis of Real-World Data in China.Front Med (Lausanne). 2021 Jun 9;8:653777. doi: 10.3389/fmed.2021.653777. eCollection 2021. Front Med (Lausanne). 2021. PMID: 34179040 Free PMC article.
-
Better response to Tanreqing injection in frequent acute exacerbation of chronic obstructive pulmonary disease (AECOPD) patients-Real-world evidence from a nationwide registry (ACURE) study.Front Pharmacol. 2023 Mar 16;14:1118143. doi: 10.3389/fphar.2023.1118143. eCollection 2023. Front Pharmacol. 2023. PMID: 37056988 Free PMC article.
-
Real-world antibiotic use in treating acute exacerbations of chronic obstructive pulmonary disease (AECOPD) in China: Evidence from the ACURE study.Front Pharmacol. 2021 May 25;12:649884. doi: 10.3389/fphar.2021.649884. eCollection 2021. Front Pharmacol. 2021. PMID: 34113250 Free PMC article.
-
Clinical Features and Outcomes of Acute Exacerbation in Chronic Obstructive Pulmonary Disease Patients with Pulmonary Heart Disease: A Multicenter Observational Study.Int J Chron Obstruct Pulmon Dis. 2021 Oct 22;16:2901-2910. doi: 10.2147/COPD.S325925. eCollection 2021. Int J Chron Obstruct Pulmon Dis. 2021. PMID: 34712043 Free PMC article.
-
Characteristics, Management and In-Hospital Clinical Outcomes Among Inpatients with Acute Exacerbation of Chronic Obstructive Pulmonary Disease in China: Results from the Phase I Data of ACURE Study.Int J Chron Obstruct Pulmon Dis. 2021 Feb 25;16:451-465. doi: 10.2147/COPD.S281957. eCollection 2021. Int J Chron Obstruct Pulmon Dis. 2021. PMID: 33658775 Free PMC article. Review.
References
-
- Bafadhel M., Peterson S., De Blas M. A., Calverley P. M., Rennard S. I., Richter K., et al. (2018). Predictors of Exacerbation Risk and Response to Budesonide in Patients with Chronic Obstructive Pulmonary Disease: a post-hoc Analysis of Three Randomised Trials. Lancet Respir. Med. 6, 117–126. 10.1016/S2213-2600(18)30006-7 - DOI - PubMed
LinkOut - more resources
Full Text Sources

