"Osteomicrobiology": The Nexus Between Bone and Bugs
- PMID: 35145499
- PMCID: PMC8822158
- DOI: 10.3389/fmicb.2021.812466
"Osteomicrobiology": The Nexus Between Bone and Bugs
Abstract
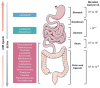
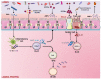
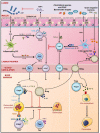
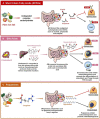
A growing body of scientific evidence supports the notion that gut microbiota plays a key role in the regulation of various physiological and pathological processes related to human health. Recent findings have now established that gut microbiota also contributes to the regulation of bone homeostasis. Studies on animal models have unraveled various underlying mechanisms responsible for gut microbiota-mediated bone regulation. Normal gut microbiota is thus required for the maintenance of bone homeostasis. However, dysbiosis of gut microbiota communities is reported to be associated with several bone-related ailments such as osteoporosis, rheumatoid arthritis, osteoarthritis, and periodontitis. Dietary interventions in the form of probiotics, prebiotics, synbiotics, and postbiotics have been reported in restoring the dysbiotic gut microbiota composition and thus could provide various health benefits to the host including bone health. These dietary interventions prevent bone loss through several mechanisms and thus could act as potential therapies for the treatment of bone pathologies. In the present review, we summarize the current knowledge of how gut microbiota and its derived microbial compounds are associated with bone metabolism and their roles in ameliorating bone health. In addition to this, we also highlight the role of various dietary supplements like probiotics, prebiotics, synbiotics, and postbiotics as promising microbiota targeted interventions with the clinical application for leveraging treatment modalities in various inflammatory bone pathologies.
Keywords: bone health; gut microbiota; osteomicrobiology; prebiotics; probiotics.
Copyright © 2022 Bhardwaj, Sapra, Tiwari, Mishra, Sharma and Srivastava.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



References
-
- Aguilar-Toalá J. E., Garcia-Varela R., Garcia H. S., Mata-Haro V., González-Córdova A. F., Vallejo-Cordoba B., et al. (2018). Postbiotics: An evolving term within the functional foods field. Trends Food Sci. Technol. 75 105–114. 10.1016/j.tifs.2018.03.009 - DOI
-
- Ahmed S., Macfarlane G. T., Fite A., McBain A. J., Gilbert P., Macfarlane S. (2007). Mucosa-associated bacterial diversity in relation to human terminal ileum and colonic biopsy samples. Appl. Environ. Microbiol. 73 7435–7442. 10.1128/AEM.01143-07/ASSET/2C475B3D-6743-4017-9D4E-7EFB9023F2BB/ASSETS/GRAPHIC/ZAM0220783380003.JPEG - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources

