Immune Response in Moderate to Critical Breakthrough COVID-19 Infection After mRNA Vaccination
- PMID: 35145522
- PMCID: PMC8821964
- DOI: 10.3389/fimmu.2022.816220
Immune Response in Moderate to Critical Breakthrough COVID-19 Infection After mRNA Vaccination
Abstract
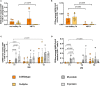
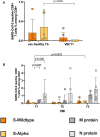
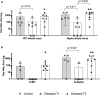
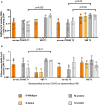
SARS-CoV-2 variants of concern (VOCs) can trigger severe endemic waves and vaccine breakthrough infections (VBI). We analyzed the cellular and humoral immune response in 8 patients infected with the alpha variant, resulting in moderate to fatal COVID-19 disease manifestation, after double mRNA-based anti-SARS-CoV-2 vaccination. In contrast to the uninfected vaccinated control cohort, the diseased individuals had no detectable high-avidity spike (S)-reactive CD4+ and CD8+ T cells against the alpha variant and wild type (WT) at disease onset, whereas a robust CD4+ T-cell response against the N- and M-proteins was generated. Furthermore, a delayed alpha S-reactive high-avidity CD4+ T-cell response was mounted during disease progression. Compared to the vaccinated control donors, these patients also had lower neutralizing antibody titers against the alpha variant at disease onset. The delayed development of alpha S-specific cellular and humoral immunity upon VBI indicates reduced immunogenicity against the S-protein of the alpha VOC, while there was a higher and earlier N- and M-reactive T-cell response. Our findings do not undermine the current vaccination strategies but underline a potential need for the inclusion of VBI patients in alternative vaccination strategies and additional antigenic targets in next-generation SARS-CoV-2 vaccines.
Keywords: SARS-CoV-2; T cells; breakthrough infection; mRNA; neutralizing antibodies; vaccine.
Copyright © 2022 Paniskaki, Anft, Meister, Marheinecke, Pfaender, Skrzypczyk, Seibert, Thieme, Konik, Dolff, Anastasiou, Holzer, Dittmer, Queren, Fricke, Rohn, Westhoff, Witzke, Stervbo, Roch and Babel.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

