Integrated Analyses of Gut Microbiome and Host Metabolome in Children With Henoch-Schönlein Purpura
- PMID: 35145922
- PMCID: PMC8821812
- DOI: 10.3389/fcimb.2021.796410
Integrated Analyses of Gut Microbiome and Host Metabolome in Children With Henoch-Schönlein Purpura
Abstract
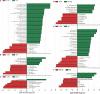
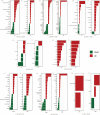
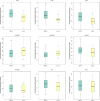
Recent studies have shown that intestinal microbes and metabolites are involved in the pathogenesis of many diseases. However, whether and how they are related to Henoch-Schönlein purpura (HSP) has yet to be understood. This work is designed to detect gut microbes, intestinal and serum metabolites in children with HSP, trying to discover the etiology and pathogenesis of HSP. A total of 86 children were recruited in this study, namely, 58 children with HSP (HSP group) and 28 healthy children as control groups (CON group). 16S rDNA amplicon sequencing technology and UPLC-QTOF/MS non-targeted metabolomics analysis were used to detect the intestinal microbes and metabolites, and also multi-reaction monitoring technology for detecting serum arachidonic acid (AA) and its metabolites. Then, correlation analysis was performed to explore the possible interaction between the differential gut microbes and metabolites. As a result, at the microbiota family level, the CON group had an advantage of Coriobacteriaceae while the HSP group had a dominant Bacteroidaceae. Five kinds of bacteria in the HSP group were significantly enriched at the genus level, and seven kinds of bacteria were significantly enriched in the CON group. A total of 59 kinds of gut metabolites significantly differ between the two groups, in which most are lipids and peptides. Spearman correlation analysis showed that Bacteroides, Dialister, and Agathobacter were associated with unsaturated fatty acids, especially AA metabolism. Then, we tested the AA related metabolites in serum and found thromboxane B2, leukotriene B4, prostaglandin D2, 9S-hydroxyoctadecadienoic acid, and 13S-hydroxyoctadecadienoic acid significantly changed. In conclusion, children with HSP had dominant Bacteroidaceae and decreased Coriobacteriaceae in the family level of gut microbes, and also lipids and peptides changed most in the gut metabolites. Our data suggested that the biosynthesis and metabolism of unsaturated fatty acids, especially AA and its metabolites, might participate in the occurrence and development of HSP.
Keywords: 16S rDNA; Arachidonic acid; Henoch-Schönlein purpura; gut microbiota; host metabolome; unsaturated fatty acid.
Copyright © 2022 Wen, Dang, Feng, He, Li, Liu and He.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Research Materials

