Silver(I)-Tazobactam Frameworks with Improved Antimicrobial Activity
- PMID: 35145956
- PMCID: PMC8822216
- DOI: 10.3389/fchem.2021.815827
Silver(I)-Tazobactam Frameworks with Improved Antimicrobial Activity
Abstract
Tazobactam (TazoH) is a penicillinate sulfone β-lactamase inhibitor with negligible antimicrobial activity, commonly used with other antibiotics to provide an effective combination against many susceptible organisms expressing β-lactamases. Two novel Ag(I)-tazobactam frameworks ([Ag(I)-Tazo] and [Ag(I)-Tazo2]) prepared by mechanochemistry are presented herein as alternative forms to improve the antimicrobial activity of tazobactam by exploring synergistic effects with silver, being the first crystal structures reported of tazobactam coordinating to a metal site. The topological analysis of the 3D ([Ag(I)-Tazo]) and 2D+1D ([Ag(I)-Tazo2]) frameworks revealed underlying nets with the cbs (CrB self-dual) and decorated sql topologies, respectively. These novel frameworks are stable and show an enhanced antimicrobial activity when compared to tazobactam alone. Amongst the tested microorganisms, Pseudomonas aeruginosa is the most sensitive to tazobactam and the new compounds. This study thus unveils novel facets of tazobactam chemistry and opens up its application as a multifunctional linker for the design of antibiotic coordination frameworks and related materials.
Keywords: antibiotic coordination frameworks; mechanochemistry; silver; supramolecular chemistry; tazobactam.
Copyright © 2022 Ferreira, Alves, Kirillov, Rijo and André.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


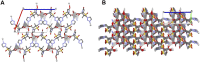







References
-
- An H., Li M., Gao J., Zhang Z., Ma S., Chen Y. (2019). Incorporation of Biomolecules in Metal-Organic Frameworks for Advanced Applications. Coord. Chem. Rev. 384, 90–106. 10.1016/j.ccr.2019.01.001 - DOI
-
- André V., Alves P. C., Duarte M. T. (2021). Exploring Antibiotics as Ligands in Metal-Organic and Hydrogen Bonding Frameworks: Our Novel Approach towards Enhanced Antimicrobial Activity (Mini-review). Inorg. Chim. Acta 525 (September), 120474. 10.1016/j.ica.2021.120474 - DOI
-
- Asai N., Suematsu H., Ohashi W., Shibata Y., Sakanashi D., Kato H., et al. (2021). Ceftriaxone versus Tazobactam/Piperacillin and Carbapenems in the Treatment of Aspiration Pneumonia: A Propensity Score Matching Analysis. J. Infect. Chemother. 27 (10), 1465–1470. 10.1016/j.jiac.2021.06.011 - DOI - PubMed
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

