Metabolic Engineering of Escherichia coli for Ectoine Production With a Fermentation Strategy of Supplementing the Amino Donor
- PMID: 35145959
- PMCID: PMC8822159
- DOI: 10.3389/fbioe.2022.824859
Metabolic Engineering of Escherichia coli for Ectoine Production With a Fermentation Strategy of Supplementing the Amino Donor
Abstract
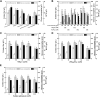
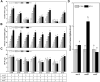
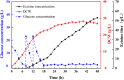
Ectoine, an osmotic pressure-compensated solute, is used in the food, agriculture, medicine, and cosmetics industries due to its ability to protect macromolecules. In this study, an ectoine-producing variant of Escherichia coli, ET08, was genetically constructed by introducing the ectABC gene cluster and eliminating metabolic pathways involving lysine and pyruvate. Medium optimization enhanced ectoine production from 1.87 to 10.2 g/L. Analysis of the transcriptional levels revealed that supplementation with ammonium sulfate enhanced the metabolic flux towards the biosynthesis of ectoine. Furthermore, by optimizing the copy number of ectA, ectB, and ectC, the recombinant E. coli ET11 (ectA:ectB:ectC = 1:2:1) produced 12.9 g/L ectoine in the shake flask and 53.2 g/L ectoine in a fed-batch fermenter, representing the highest ectoine titer produced by E. coli, which has great industrial prospects.
Keywords: Escherichia coli; amino donor; ectoine; medium optimization; metabolic engineering.
Copyright © 2022 Zhang, Liang, Zhao, Ma, Luo, Li and Xu.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



Similar articles
-
High Ectoine Production from Lignocellulosic Hydrolysate by Escherichia coli through Metabolic and Fermentation Engineering.ACS Synth Biol. 2025 Feb 21;14(2):609-620. doi: 10.1021/acssynbio.4c00899. Epub 2025 Feb 11. ACS Synth Biol. 2025. PMID: 39933098
-
Metabolic Engineering of Escherichia coli for Efficient Production of Ectoine.J Agric Food Chem. 2025 Jan 8;73(1):646-654. doi: 10.1021/acs.jafc.4c07640. Epub 2024 Dec 26. J Agric Food Chem. 2025. PMID: 39723826
-
Multistep Metabolic Engineering of Escherichia coli for High-Level Ectoine Production.ACS Synth Biol. 2025 Apr 18;14(4):1230-1239. doi: 10.1021/acssynbio.4c00876. Epub 2025 Mar 25. ACS Synth Biol. 2025. PMID: 40131136
-
Genes and enzymes of ectoine biosynthesis in halotolerant methanotrophs.Methods Enzymol. 2011;495:15-30. doi: 10.1016/B978-0-12-386905-0.00002-4. Methods Enzymol. 2011. PMID: 21419912 Review.
-
Biotechnological production of ectoine: current status and prospects.Folia Microbiol (Praha). 2024 Apr;69(2):247-258. doi: 10.1007/s12223-023-01105-4. Epub 2023 Nov 14. Folia Microbiol (Praha). 2024. PMID: 37962826 Review.
Cited by
-
High production of ectoine from methane in genetically engineered Methylomicrobium alcaliphilum 20Z by preventing ectoine degradation.Microb Cell Fact. 2024 May 2;23(1):127. doi: 10.1186/s12934-024-02404-2. Microb Cell Fact. 2024. PMID: 38698430 Free PMC article.
-
Comparative genomic analysis of Halomonas campaniensis wild-type and ultraviolet radiation-mutated strains reveal genomic differences associated with increased ectoine production.Int Microbiol. 2023 Nov;26(4):1009-1020. doi: 10.1007/s10123-023-00356-y. Epub 2023 Apr 17. Int Microbiol. 2023. PMID: 37067733 Free PMC article.
-
Improved Productivity of Streptomyces mobaraensis Transglutaminase by Regulating Zymogen Activation.Front Bioeng Biotechnol. 2022 Apr 14;10:878795. doi: 10.3389/fbioe.2022.878795. eCollection 2022. Front Bioeng Biotechnol. 2022. PMID: 35497347 Free PMC article.
-
Multiple Functions of Compatible Solute Ectoine and Strategies for Constructing Overproducers for Biobased Production.Mol Biotechnol. 2024 Aug;66(8):1772-1785. doi: 10.1007/s12033-023-00827-7. Epub 2023 Jul 24. Mol Biotechnol. 2024. PMID: 37488320 Review.
-
Improved salt tolerance of Synechococcus elongatus PCC 7942 by heterologous synthesis of compatible solute ectoine.Front Microbiol. 2023 Feb 2;14:1123081. doi: 10.3389/fmicb.2023.1123081. eCollection 2023. Front Microbiol. 2023. PMID: 36819058 Free PMC article.
References
-
- Bursy J., Kuhlmann A. U., Pittelkow M., Hartmann H., Jebbar M., Pierik A. J., et al. (2008). Synthesis and Uptake of the Compatible Solutes Ectoine and 5-hydroxyectoine by Streptomyces Coelicolor A3(2) in Response to Salt and Heat Stresses. Appl. Environ. Microbiol. 74, 7286–7296. 10.1128/aem.00768-08 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

