Monocyte-Platelet Aggregates Triggered by CD31 Molecule in Non-ST Elevation Myocardial Infarction: Clinical Implications in Plaque Rupture
- PMID: 35146002
- PMCID: PMC8821091
- DOI: 10.3389/fcvm.2021.741221
Monocyte-Platelet Aggregates Triggered by CD31 Molecule in Non-ST Elevation Myocardial Infarction: Clinical Implications in Plaque Rupture
Abstract
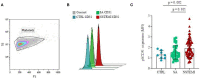
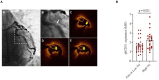
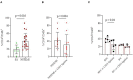
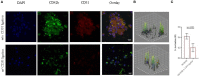
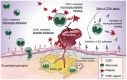
Despite the recent innovations in cardiovascular care, atherothrombosis is still a major complication of acute coronary syndromes (ACS). We evaluated the involvement of the CD31 molecule in thrombotic risk through the formation of monocyte-platelet (Mo-Plt) aggregates in patients with ACS with no-ST-segment elevation myocardial infarction (NSTEMI) on top of dual anti-platelet therapy (DAPT). We enrolled 19 control (CTRL) subjects, 46 stable angina (SA), and 86 patients with NSTEMI, of which, 16 with Intact Fibrous Cap (IFC) and 19 with Ruptured Fibrous Cap (RFC) as assessed by the Optical Coherence Tomography (OCT). The expression of CD31 on monocytes and platelets was measured. Following the coronary angiography, 52 NSTEMIs were further stratified according to thrombus grade (TG) evaluation. Finally, a series of ex vivo experiments verified whether the CD31 participates in Mo-Plt aggregate formation. In patients with NSTEMI, CD31 was reduced on monocytes and was increased on platelets, especially in NSTEMI presented with RFC plaques compared to those with IFC lesions, and in patients with high TG compared to those with zero/low TG. Ex vivo experiments documented an increase in Mo-Plt aggregates among NSTEMI, which significantly decreased after the CD31 ligation, particularly in patients with RFC plaques. In NSTEMI, CD31 participates in Mo-Plt aggregate formation in spite of optimal therapy and DAPT, suggesting the existence of alternative thrombotic pathways, as predominantly displayed in patients with RFC.
Keywords: CD31; acute coronary syndromes; monocyte-platelet aggregates; plaque rupture; precision medicine; thrombus burden; unstable plaque.
Copyright © 2022 Vinci, Pedicino, Bonanni, d'Aiello, Pisano, Ponzo, Severino, Ciampi, Canonico, Russo, Di Sario, Vergallo, Filomia, Montone, Flego, Stefanini, Piacentini, Conte, Cribari, Massetti, Crea and Liuzzo.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


Similar articles
-
Correlation between CD4+CD28null T lymphocytes, regulatory T cells and plaque rupture: An Optical Coherence Tomography study in Acute Coronary Syndromes.Int J Cardiol. 2019 Feb 1;276:289-292. doi: 10.1016/j.ijcard.2018.08.101. Epub 2018 Sep 7. Int J Cardiol. 2019. PMID: 30217424
-
Coronary CT angiographic characteristics of culprit lesions in acute coronary syndromes not related to plaque rupture as defined by optical coherence tomography and angioscopy.Eur Heart J. 2011 Nov;32(22):2814-23. doi: 10.1093/eurheartj/ehr189. Epub 2011 Jun 30. Eur Heart J. 2011. PMID: 21719455
-
Coronary plaque rupture with subsequent thrombosis typifies the culprit lesion of non-ST-segment-elevation myocardial infarction, not unstable angina: non-ST-segment-elevation acute coronary syndrome study.Heart Vessels. 2017 Mar;32(3):241-251. doi: 10.1007/s00380-016-0862-6. Epub 2016 Jun 21. Heart Vessels. 2017. PMID: 27325227
-
Contemporary NSTEMI management: the role of the hospitalist.Hosp Pract (1995). 2020 Feb;48(1):1-11. doi: 10.1080/21548331.2020.1701329. Epub 2020 Feb 20. Hosp Pract (1995). 2020. PMID: 31815570 Review.
-
API expert consensus document on management of ischemic heart disease.J Assoc Physicians India. 2006 Jun;54:469-80. J Assoc Physicians India. 2006. PMID: 16909697 Review.
Cited by
-
Top of basilar syndrome due to vertebral artery dissection: How high-resolution MRI and CD31 analysis of thrombus could help.Int J Surg Case Rep. 2023 Nov;112:108948. doi: 10.1016/j.ijscr.2023.108948. Epub 2023 Oct 10. Int J Surg Case Rep. 2023. PMID: 37832359 Free PMC article.
-
Targeting Inflammatory Pathways in Atherosclerosis: Exploring New Opportunities for Treatment.Curr Atheroscler Rep. 2024 Dec;26(12):707-719. doi: 10.1007/s11883-024-01241-3. Epub 2024 Oct 15. Curr Atheroscler Rep. 2024. PMID: 39404934 Free PMC article. Review.
-
Dysregulation of immune cells and platelet-monocyte aggregates in chronic thromboembolic pulmonary hypertension.Respir Res. 2025 Jul 12;26(1):245. doi: 10.1186/s12931-025-03284-9. Respir Res. 2025. PMID: 40652206 Free PMC article.
References
-
- Collet JP, Thiele H, Barbato E, Barthélémy O, Bauersachs J, Bhatt DL, et al. . ESC scientific document group. 2020 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation. Eur Heart J. (2021) 42:1289–367. 10.1093/eurheartj/ehab088 - DOI - PubMed
-
- Valgimigli M, Bueno H, Byrne RA, Collet JP, Costa F, Jeppsson A, et al. . ESC Scientific Document Group; ESC Committee for Practice Guidelines (CPG); ESC National Cardiac Societies. 2017 ESC focused update on dual antiplatelet therapy in coronary artery disease developed in collaboration with EACTS: The Task Force for dual antiplatelet therapy in coronary artery disease of the European Society of Cardiology (ESC) and of the European Association for Cardio-Thoracic Surgery (EACTS). Eur Heart J. (2018) 39:213–60. 10.1093/eurheartj/ehx638 - DOI - PubMed
-
- Benenati S, Galli M, Marzo V, Pescetelli F, Toma M, Andreotti F., et al. . Very short vs. long dual antiplatelet therapy after second generation drug-eluting stents in 35 785 patients undergoing percutaneous coronary interventions: a meta-analysis of randomized controlled trials. Eur Heart J Cardiovasc Pharmacother. (2021) 7:86–93. 10.1093/ehjcvp/pvaa001 - DOI - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

