Nuclear lamins: Structure and function in mechanobiology
- PMID: 35146235
- PMCID: PMC8810204
- DOI: 10.1063/5.0082656
Nuclear lamins: Structure and function in mechanobiology
Abstract
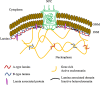
Nuclear lamins are type V intermediate filament proteins that polymerize into complex filamentous meshworks at the nuclear periphery and in less structured forms throughout the nucleoplasm. Lamins interact with a wide range of nuclear proteins and are involved in numerous nuclear and cellular functions. Within the nucleus, they play roles in chromatin organization and gene regulation, nuclear shape, size, and mechanics, and the organization and anchorage of nuclear pore complexes. At the whole cell level, they are involved in the organization of the cytoskeleton, cell motility, and mechanotransduction. The expression of different lamin isoforms has been associated with developmental progression, differentiation, and tissue-specific functions. Mutations in lamins and their binding proteins result in over 15 distinct human diseases, referred to as laminopathies. The laminopathies include muscular (e.g., Emery-Dreifuss muscular dystrophy and dilated cardiomyopathy), neurological (e.g., microcephaly), and metabolic (e.g., familial partial lipodystrophy) disorders as well as premature aging diseases (e.g., Hutchinson-Gilford Progeria and Werner syndromes). How lamins contribute to the etiology of laminopathies is still unknown. In this review article, we summarize major recent findings on the structure, organization, and multiple functions of lamins in nuclear and more global cellular processes.
© 2022 Author(s).
Figures




References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

