Thoracic Radiotherapy in Limited-Stage SCLC-a Population-Based Study of Patterns of Care in Norway From 2000 Until 2018
- PMID: 35146461
- PMCID: PMC8801751
- DOI: 10.1016/j.jtocrr.2021.100270
Thoracic Radiotherapy in Limited-Stage SCLC-a Population-Based Study of Patterns of Care in Norway From 2000 Until 2018
Abstract
Introduction: Twice-daily (BID) thoracic radiotherapy (TRT) of 45 Gy per 30 fractions is recommended for limited-stage (LS) SCLC, but most patients are treated with once-daily (OD) schedules owing to toxicity concerns and logistic challenges. An alternative is hypofractionated OD TRT of 40 to 42 Gy per 15 fractions. A randomized trial by our group indicated that TRT of 45 Gy per 30 fractions is more effective than TRT of 42 Gy per 15 fractions, and because it was not more toxic, 45 BID replaced 42 OD as the recommended schedule in Norway. The aims of this study were to evaluate to what extent BID TRT has been implemented in Norway and whether this practice change has led to improved survival.
Methods: Data on all patients diagnosed with LS SCLC from 2000 until 2018 were collected from the Cancer Registry of Norway, containing nearly complete data on cancer diagnosis, radiotherapy, and survival.
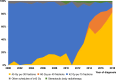
Results: A total of 2222 patients were identified; median age was 69 years, 51.8% were women, and 87.1% had stage II to III disease. Overall, 64.6% received TRT. The use of BID TRT increased from 1.8% (2000-2004) to 83.2% (2015-2018). Median overall survival among patients receiving curative TRT improved significantly during the study period (2000-2004: 17.9 mo, 2015-2018: 25.0 mo, p = 0.0023), and patients receiving 45 BID had significantly longer median overall survival than patients receiving 42 OD (BID: 26.2 mo, OD: 19.6 mo, p = 0.0015).
Conclusions: BID TRT has replaced hypofractionated OD TRT as the standard treatment of LS SCLC in Norway which has led to a significant (p = 0.0023) and clinically relevant survival improvement.
Keywords: Accelerated; Hyperfractionated; Hypofractionated; Radiotherapy schedule; Survival; Twice-daily.
© 2021 The Authors.
Figures


References
-
- Govindan R., Page N., Morgensztern D., et al. Changing epidemiology of small-cell lung cancer in the United States over the last 30 years: analysis of the surveillance, epidemiologic, and end results database. J Clin Oncol. 2006;24:4539–4544. - PubMed
-
- Cancer registry of Norway. Lung cancer annual report. https://www.kreftregisteret.no/globalassets/publikasjoner-og-rapporter/a...
-
- Murray N., Turrisi A.T., 3rd A review of first-line treatment for small-cell lung cancer. J Thorac Oncol. 2006;1:270–278. - PubMed
-
- Sundstrøm S., Bremnes R.M., Kaasa S., et al. Cisplatin and etoposide regimen is superior to cyclophosphamide, epirubicin, and vincristine regimen in small-cell lung cancer: results from a randomized phase III trial with 5 years’ follow-up. J Clin Oncol. 2002;20:4665–4672. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

