Effect of heterogeneous substrate adhesivity of follower cells on speed and tension profile of leader cells in primary keratocyte collective cell migration
- PMID: 35146504
- PMCID: PMC8918985
- DOI: 10.1242/bio.058893
Effect of heterogeneous substrate adhesivity of follower cells on speed and tension profile of leader cells in primary keratocyte collective cell migration
Abstract
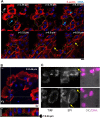
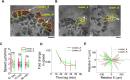
In single keratocyte motility, membrane tension is reported to be high at cell-fronts and believed to establish front coherence. To understand role of membrane mechanics in collective cell migration, we study membrane height fluctuations in cell sheets from fish scales using interference reflection microscopy (IRM). We report the monolayer to have cells lacking substrate adhesion and show that such 'non-sticky' cells can form bridges between leader cells and far-away follower cells. Do such interactions alter motility and membrane mechanics in such leaders? We find non-significant, but reduced speed for leaders with 'non-sticky' followers in comparison to other leaders. Cells show high phenotypic variability in their membrane fluctuation tension profiles. On average, this tension is found to be lower at cell fronts than the mid-section. However, leaders with non-sticky followers are more prone to display higher tension at their front and have a negative correlation between cell speed and front-mid tension difference. Thus, we conclude that intracellular tension gradients are heterogeneous in cell sheets and substrate adhesivity of followers can control the coupling of the gradient to cell speed.
Keywords: Adhesion; Collective cell migration; Membrane fluctuations; Tension gradient.
© 2022. Published by The Company of Biologists Ltd.
Conflict of interest statement
Competing interests The authors declare no competing or financial interests.
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

