Morphologies, dimensions and targets of gastric nitric oxide synthase neurons
- PMID: 35146560
- PMCID: PMC8976817
- DOI: 10.1007/s00441-022-03594-0
Morphologies, dimensions and targets of gastric nitric oxide synthase neurons
Abstract
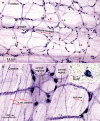
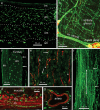
We investigated the distributions and targets of nitrergic neurons in the rat stomach, using neuronal nitric oxide synthase (NOS) immunohistochemistry and nicotinamide adenine dinucleotide phosphate (NADPH) diaphorase histochemistry. Nitrergic neurons comprised similar proportions of myenteric neurons, about 30%, in all gastric regions. Small numbers of nitrergic neurons occurred in submucosal ganglia. In total, there were ~ 125,000 neuronal nitric oxide synthase (nNOS) neurons in the stomach. The myenteric cell bodies had single axons, type I morphology and a wide range of sizes. Five targets were identified, the longitudinal, circular and oblique layers of the external muscle, the muscularis mucosae and arteries within the gastric wall. The circular and oblique muscle layers had nitrergic fibres throughout their thickness, while the longitudinal muscle was innervated at its inner surface by fibres of the tertiary plexus, a component of the myenteric plexus. There was a very dense innervation of the pyloric sphincter, adjacent to the duodenum. The muscle strands that run between mucosal glands rarely had closely associated nNOS nerve fibres. Both nNOS immunohistochemistry and NADPH histochemistry showed that nitrergic terminals did not provide baskets of terminals around myenteric neurons. Thus, the nitrergic neuron populations in the stomach supply the muscle layers and intramural arteries, but, unlike in the intestine, gastric interneurons do not express nNOS. The large numbers of nNOS neurons and the density of innervation of the circular muscle and pyloric sphincter suggest that there is a finely graded control of motor function in the stomach by the recruitment of different numbers of inhibitory motor neurons.
Keywords: Enteric nervous system; Gastric motor neurons; Inhibitory neurons; Nitrergic neurons; Nitric oxide; Stomach.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures






Similar articles
-
Nitric oxide synthase immunoreactivity in the enteric nervous system of the developing human digestive tract.Cell Tissue Res. 1994 Feb;275(2):235-45. doi: 10.1007/BF00319421. Cell Tissue Res. 1994. PMID: 7509262
-
Neuronal nitric oxide synthase activity is increased during granulomatous inflammation in the colon and caecum of pigs infected with Schistosoma japonicum.Auton Neurosci. 2002 Jul 31;99(1):1-12. doi: 10.1016/s1566-0702(02)00042-5. Auton Neurosci. 2002. PMID: 12171250
-
Nitric oxide synthase in the enteric nervous system of the guinea-pig: a quantitative description.Cell Tissue Res. 1994 Jul;277(1):139-49. doi: 10.1007/BF00303090. Cell Tissue Res. 1994. PMID: 7519970
-
Pathophysiological significance of neuronal nitric oxide synthase in the gastrointestinal tract.J Gastroenterol. 2003;38(5):421-30. doi: 10.1007/s00535-003-1094-y. J Gastroenterol. 2003. PMID: 12768383 Review.
-
Nitric oxide and its role as a non-adrenergic, non-cholinergic inhibitory neurotransmitter in the gastrointestinal tract.Br J Pharmacol. 2019 Jan;176(2):212-227. doi: 10.1111/bph.14459. Epub 2018 Sep 3. Br J Pharmacol. 2019. PMID: 30063800 Free PMC article. Review.
Cited by
-
Ex Vivo Study of Colon Health, Contractility and Innervation in Male and Female Rats after Regular Exposure to Instant Cascara Beverage.Foods. 2024 Aug 6;13(16):2474. doi: 10.3390/foods13162474. Foods. 2024. PMID: 39200401 Free PMC article.
-
Diffeomorphic Surface Modeling for MRI-Based Characterization of Gastric Anatomy and Motility.IEEE Trans Biomed Eng. 2023 Jul;70(7):2046-2057. doi: 10.1109/TBME.2023.3234509. Epub 2023 Jun 20. IEEE Trans Biomed Eng. 2023. PMID: 37018592 Free PMC article.
-
Characterization of neuromuscular transmission and projections of muscle motor neurons in the rat stomach.Am J Physiol Gastrointest Liver Physiol. 2024 Jan 1;326(1):G78-G93. doi: 10.1152/ajpgi.00194.2023. Epub 2023 Nov 21. Am J Physiol Gastrointest Liver Physiol. 2024. PMID: 37987773 Free PMC article.
-
Immunohistochemical characterization of interstitial cells and their spatial relationship to motor neurons within the mouse esophagus.Cell Tissue Res. 2025 Jan;399(1):61-84. doi: 10.1007/s00441-024-03929-z. Epub 2024 Nov 28. Cell Tissue Res. 2025. PMID: 39607495
References
-
- Boeckxstaens GE, Pelckmans PA, Bogers JJ, Bult H, De Man JG, Oosterbosch L, Herman AG, Van Maercke YM. Release of nitric oxide upon stimulation of nonadrenergic noncholinergic nerves in the rat gastric fundus. J Pharmacol Exp Ther. 1991;256:441–447. - PubMed
-
- Boeckxstaens GE, Pelckmans PA, De Man JG, Bult H, Herman AG, Van Maercke YM. Evidence for a differential release of nitric oxide and vasoactive intestinal polypeptide by noradrenic noncholinergic nerves in the rat gastric fundus. Arch Int Pharmacodyn Ther. 1992;318:107–115. - PubMed
-
- Bossolani GDP, Pintelon I, Detrez JD, Buckinx R, Thys S, Zanoni JN, De Vos WH, Timmermans J-P (2018) Comparative analysis reveals Ce3D as optimal clearing method for in toto imaging of the mouse intestine. Neurogastroenterol Motil 31:e13560 - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

