Meta-Analysis of Drug Delivery Approaches for Treating Intracellular Infections
- PMID: 35146592
- PMCID: PMC8830998
- DOI: 10.1007/s11095-022-03188-z
Meta-Analysis of Drug Delivery Approaches for Treating Intracellular Infections
Abstract
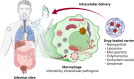
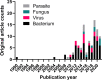
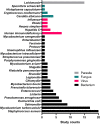
This meta-analysis aims to evaluate the trend, methodological quality and completeness of studies on intracellular delivery of antimicrobial agents. PubMed, Embase, and reference lists of related reviews were searched to identify original articles that evaluated carrier-mediated intracellular delivery and pharmacodynamics (PD) of antimicrobial therapeutics against intracellular pathogens in vitro and/or in vivo. A total of 99 studies were included in the analysis. The most commonly targeted intracellular pathogens were bacteria (62.6%), followed by viruses (16.2%) and parasites (15.2%). Twenty-one out of 99 (21.2%) studies performed neither microscopic imaging nor flow cytometric analysis to verify that the carrier particles are present in the infected cells. Only 31.3% of studies provided comparative inhibitory concentrations against a free drug control. Approximately 8% of studies, albeit claimed for intracellular delivery of antimicrobial therapeutics, did not provide any experimental data such as microscopic imaging, flow cytometry, and in vitro PD. Future research on intracellular delivery of antimicrobial agents needs to improve the methodological quality and completeness of supporting data in order to facilitate clinical translation of intracellular delivery platforms for antimicrobial therapeutics.
Keywords: Antimicrobials; Drug carriers; Intracellular drug delivery; Intracellular pathogens.
© 2022. The Author(s), under exclusive licence to Springer Science+Business Media, LLC, part of Springer Nature.
Figures

Similar articles
-
Delivery of Antibiotics by Cell-Penetrating Peptides to Kill Intracellular Pathogens.Methods Mol Biol. 2022;2383:335-345. doi: 10.1007/978-1-0716-1752-6_22. Methods Mol Biol. 2022. PMID: 34766300
-
Nanotherapeutic provides dose sparing and improved antimicrobial activity against Brucella melitensis infections.J Control Release. 2019 Jan 28;294:288-297. doi: 10.1016/j.jconrel.2018.12.024. Epub 2018 Dec 17. J Control Release. 2019. PMID: 30572034
-
Bioinspired drug delivery strategies for repurposing conventional antibiotics against intracellular infections.Adv Drug Deliv Rev. 2021 Oct;177:113948. doi: 10.1016/j.addr.2021.113948. Epub 2021 Aug 28. Adv Drug Deliv Rev. 2021. PMID: 34464665 Review.
-
Challenges and solutions in polymer drug delivery for bacterial biofilm treatment: A tissue-by-tissue account.Adv Drug Deliv Rev. 2021 Nov;178:113973. doi: 10.1016/j.addr.2021.113973. Epub 2021 Sep 13. Adv Drug Deliv Rev. 2021. PMID: 34530014 Review.
-
A bioinspired hierarchical nanoplatform targeting and responding to intracellular pathogens to eradicate parasitic infections.Biomaterials. 2022 Jan;280:121309. doi: 10.1016/j.biomaterials.2021.121309. Epub 2021 Dec 6. Biomaterials. 2022. PMID: 34896862
Cited by
-
Editorial of Special Issue "Cytoplasmic Delivery of Bioactives".Pharm Res. 2022 Jun;39(6):1031-1034. doi: 10.1007/s11095-022-03290-2. Epub 2022 May 23. Pharm Res. 2022. PMID: 35606599 Free PMC article. No abstract available.
-
Effective Treatments of UTI-Is Intravesical Therapy the Future?Pathogens. 2023 Mar 6;12(3):417. doi: 10.3390/pathogens12030417. Pathogens. 2023. PMID: 36986339 Free PMC article. Review.
-
A new evidence-based design-of-experiments approach for optimizing drug delivery systems with exemplification by emulsion-derived Vancomycin-loaded PLGA capsules.Sci Rep. 2024 Dec 28;14(1):31164. doi: 10.1038/s41598-024-82496-3. Sci Rep. 2024. PMID: 39732761 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

