Active Mechanical Threading by a Molecular Motor
- PMID: 35146857
- PMCID: PMC9314141
- DOI: 10.1002/anie.202201882
Active Mechanical Threading by a Molecular Motor
Abstract
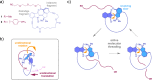
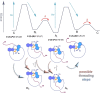
Molecular motors transform external energy input into directional motions and offer exquisite precision for nano-scale manipulations. To make full use of molecular motor capacities, their directional motions need to be transmitted and used for powering downstream molecular events. Here we present a macrocyclic molecular motor structure able to perform repetitive molecular threading of a flexible tetraethylene glycol chain through the macrocycle. This mechanical threading event is actively powered by the motor and leads to a direct translation of the unidirectional motor rotation into unidirectional translation motion (chain versus ring). The mechanism of the active mechanical threading is elucidated and the actual threading step is identified as a combined helix inversion and threading event. The established molecular machine function resembles the crucial step of macroscopic weaving or sewing processes and therefore offers a first entry point to a "molecular knitting" counterpart.
Keywords: Hemithioindigo; Indigoids; Molecular Machines; Molecular Motors; Photochemistry.
© 2022 The Authors. Angewandte Chemie International Edition published by Wiley-VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Directionality Reversal and Shift of Rotational Axis in a Hemithioindigo Macrocyclic Molecular Motor.J Am Chem Soc. 2024 Aug 21;146(33):23387-23397. doi: 10.1021/jacs.4c06377. Epub 2024 Aug 7. J Am Chem Soc. 2024. PMID: 39109636 Free PMC article.
-
Defining Unidirectional Motions and Structural Reconfiguration in a Macrocyclic Molecular Motor.J Am Chem Soc. 2023 Jun 21;145(24):13081-13088. doi: 10.1021/jacs.3c01567. Epub 2023 Jun 6. J Am Chem Soc. 2023. PMID: 37279894 Free PMC article.
-
Transmission of Unidirectional Molecular Motor Rotation to a Remote Biaryl Axis.Angew Chem Int Ed Engl. 2018 Aug 20;57(34):11064-11068. doi: 10.1002/anie.201804716. Epub 2018 Jul 18. Angew Chem Int Ed Engl. 2018. PMID: 29932486
-
Design and Synthesis of Nonequilibrium Systems.ACS Nano. 2015 Sep 22;9(9):8672-88. doi: 10.1021/acsnano.5b03809. Epub 2015 Aug 17. ACS Nano. 2015. PMID: 26222543 Review.
-
The Physics and Physical Chemistry of Molecular Machines.Chemphyschem. 2016 Jun 17;17(12):1719-41. doi: 10.1002/cphc.201600184. Epub 2016 Jun 15. Chemphyschem. 2016. PMID: 27149926 Free PMC article. Review.
Cited by
-
Photopharmacology of Antimitotic Agents.Int J Mol Sci. 2022 May 18;23(10):5657. doi: 10.3390/ijms23105657. Int J Mol Sci. 2022. PMID: 35628467 Free PMC article. Review.
-
Coupling Rotary Motion to Helicene Inversion within a Molecular Motor.Angew Chem Int Ed Engl. 2025 Jan 21;64(4):e202416097. doi: 10.1002/anie.202416097. Epub 2024 Nov 26. Angew Chem Int Ed Engl. 2025. PMID: 39526696 Free PMC article.
-
Controlling Swing Rates in Macrocyclic Molecular Mortise Hinges.Chemistry. 2023 Aug 4;29(44):e202300987. doi: 10.1002/chem.202300987. Epub 2023 Jul 7. Chemistry. 2023. PMID: 37229593 Free PMC article.
-
DNA-Based Molecular Machines: Controlling Mechanisms and Biosensing Applications.Biosensors (Basel). 2024 May 8;14(5):236. doi: 10.3390/bios14050236. Biosensors (Basel). 2024. PMID: 38785710 Free PMC article. Review.
-
Directionality Reversal and Shift of Rotational Axis in a Hemithioindigo Macrocyclic Molecular Motor.J Am Chem Soc. 2024 Aug 21;146(33):23387-23397. doi: 10.1021/jacs.4c06377. Epub 2024 Aug 7. J Am Chem Soc. 2024. PMID: 39109636 Free PMC article.
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

