Causes of mortality in elderly UICC stage III colon cancer (CC) patients--Tumor-related death and competing risks from the German AIO colorectal study group Colopredict Plus (CPP) registry
- PMID: 35146948
- PMCID: PMC9041084
- DOI: 10.1002/cam4.4540
Causes of mortality in elderly UICC stage III colon cancer (CC) patients--Tumor-related death and competing risks from the German AIO colorectal study group Colopredict Plus (CPP) registry
Abstract
Background: Colon cancer (CC) is a disease of elderly patients (pts.) with a median age of 73 years (yrs.). Lack of data about the effects of adjuvant chemotherapy (ACT) is caused by underrepresentation of this clinically relevant cohort in interventional trials. We analyzed real-world data from the German CPP registry with regard to a possible benefit of ACT in elderly (70+ yrs.) versus younger pts. (50 to <70 yrs.) taking cause-specific deaths into account.
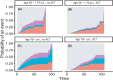
Methods: We analyzed the effect of age and ACT on overall survival (OS) and cause-specific death of stage III pts. using Cox regression.
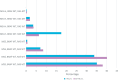
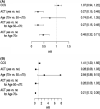
Results: In total, 1558 pts. were analyzed and follow-up was 24.6 months. 62.6% of the elderly received ACT whereas 91.1% of younger pts. (p < 0.001). Oxaliplatin combinations were significantly less often given to older than younger pts. (38.8% vs. 88.9%; p < 0.001). Mean Charlson comorbidity score was significantly lower in pts. that received ACT (0.61) than in those without ACT (1.16; p < 0.001). ACT was an independent positive prognostic factor for cancer-related death in elderly pts. even in pts. 75+ yrs. No significant difference in the effect of ACT could be observed between age groups (interaction: cancer-specific death HR = 1.7948, p = 0.1079; death of other cause HR = 0.7384, p = 0.6705).
Conclusion: ACT was an independent positive prognostic factor for OS. There may be a cohort of elderly with less co-morbidities who benefit from ACT.
Keywords: adjuvant treatment; early colon cancer; elderly; tumor-related death.
© 2022 The Authors. Cancer Medicine published by John Wiley & Sons Ltd.
Conflict of interest statement
Stefanie Nöpel Dünnebacke: Honoraria/Advisory Boards: BMS, Merck, and Roche. Anke Reinacher‐Schick: Honoraria: Amgen, AstraZeneca, BMS, Celgene, Lilly, Merck Serono, MSD, Pfizer, Roche, Sanofi‐Aventis, Servier, Advisory Boards: Amgen, AstraZeneca, BMS, Celgene, Lilly, Merck Serono, MSD, Pfizer, Roche, Sanofi‐Aventis, Servier, Baxalta, Pierre‐Fabre, Trial support: Amgen, AstraZeneca, Celgene, Ipsen, Lilly, Roche, Servier, and Mologen Berlin. Andrea Tannapfel: Honoraria/Advisory Board/ Trial Support: Roche, Amgen, Falk, and Celgene.
Figures


References
-
- Barnes B, Kraywinkel K, Kurth BM, et al. Bericht zum Krebsgeschehen in Deutschland 2016 (ed Institut RK), pp. 28–29.
-
- André T, Boni C, Mounedji‐Boudiaf L, et al. Multicenter International Study of Oxaliplatin/5‐Fluorouracil/Leucovorin in the Adjuvant Treatment of Colon Cancer (MOSAIC) Investigators. Oxaliplatin, fluorouracil, and leucovorin as adjuvant treatment for colon cancer. N Engl J Med. 2004;350(23):2343‐2351. doi: 10.1056/NEJMoa032709. PMID: 15175436. - DOI - PubMed
-
- André T, Meyerhardt J, Iveson T, et al. Effect of duration of adjuvant chemotherapy for patients with stage III colon cancer (IDEA collaboration): final results from a prospective, pooled analysis of six randomised, phase 3 trials. Lancet Oncol. 2020;21(12):1620‐1629. doi: 10.1016/S1470-2045(20)30527-1 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources

