Chemo- and mechanosensing by dendritic cells facilitate antigen surveillance in the spleen
- PMID: 35147233
- PMCID: PMC8852366
- DOI: 10.1111/imr.13055
Chemo- and mechanosensing by dendritic cells facilitate antigen surveillance in the spleen
Abstract
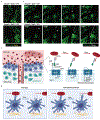
Spleen dendritic cells (DC) are critical for initiation of adaptive immune responses against blood-borne invaders. Key to DC function is their positioning at sites of pathogen entry, and their abilities to selectively capture foreign antigens and promptly engage T cells. Focusing on conventional DC2 (cDC2), we discuss the contribution of chemoattractant receptors (EBI2 or GPR183, S1PR1, and CCR7) and integrins to cDC2 positioning and function. We give particular attention to a newly identified role in cDC2 for adhesion G-protein coupled receptor E5 (Adgre5 or CD97) and its ligand CD55, detailing how this mechanosensing system contributes to splenic cDC2 positioning and homeostasis. Additional roles of CD97 in the immune system are reviewed. The ability of cDC2 to be activated by circulating missing self-CD47 cells and to integrate multiple red blood cell (RBC)-derived inputs is discussed. Finally, we describe the process of activated cDC2 migration to engage and prime helper T cells. Throughout the review, we consider the insights into cDC function in the spleen that have emerged from imaging studies.
Keywords: cell surface molecules; cell trafficking; chemokines; dendritic cells; integrins; spleen.
© 2022 John Wiley & Sons A/S. Published by John Wiley & Sons Ltd.
Conflict of interest statement
Conflict of Interest
J.G.C. is a Scientific Advisory Board member of MiroBio and BeBio and owns stock in ALX Oncology. The other authors have no financial or personal relationships that could be viewed as a conflict of interest.
Figures


References
-
- Mebius RE, Kraal G. Structure and function of the spleen. Nat Rev Immunol.2005;5:606–616. - PubMed
-
- Yamamoto K, Kobayashi T, Murakami T. Arterial terminals in the rat spleen as demonstrated by scanning electron microscopy of vascular casts. Scan Electron Microsc.1982;Pt 1:455–458. - PubMed
-
- Schmidt EE, MacDonald IC, Groom AC. Comparative aspects of splenic microcirculatory pathways in mammals: the region bordering the white pulp. Scanning Microsc.1993;7:613–628. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

