EGFR-Targeted ImmunoPET of UMUC3 Orthotopic Bladder Tumors
- PMID: 35147837
- PMCID: PMC10187976
- DOI: 10.1007/s11307-022-01708-2
EGFR-Targeted ImmunoPET of UMUC3 Orthotopic Bladder Tumors
Abstract
Purpose: Immuno-positron emission tomography (immunoPET) combines the specificity of an antibody with the sensitivity of PET to image dysregulated pathways in cancer. This study examines the performance of immunoPET using the radioimmunoconjugate [89Zr]Zr-DFO-Panitumumab to detect epidermal growth factor receptor (EGFR) expression in an orthotopic model of bladder cancer (BCa).
Procedures: Expression and quantification of EGFR receptors were confirmed in four different BCa cell lines. Binding assays validated [89Zr]Zr-DFO-Panitumumab specificity for EGFR-expressing UMUC3 BCa cells. Subcutaneous and orthotopic UMUC3 xenografts were then used for PET imaging and ex vivo biodistribution of the radioimmunoconjugate. Control cohorts included non-tumor mice, 89Zr-labeled non-specific IgG, and blocking experiments.
Results: [89Zr]Zr-DFO-Panitumumab binds specifically to EGFR-expressing UMUC3 cells with a Bmax value of 5.9 × 104 EGFRs/cell in vitro. ImmunoPET/CT images show localization of the antibody in subcutaneous UMUC3 xenografts and murine bladder tumors. In the orthotopic model, the immunoPET signal correlates with the respective tumor volume. Ex vivo biodistribution analysis further confirmed imaging results.
Conclusion: The preclinical data presents a proof of concept for utilizing EGFR-targeted immunoPET to image BCa with altered EGFR protein levels.
Keywords: Bladder Cancer; EGFR; ImmunoPET.
© 2022. World Molecular Imaging Society.
Conflict of interest statement
CONFLICT OF INTEREST STATEMENT
J. S. Lewis is an Editor-in-Chief for the Molecular Imaging and Biology journal. He recused himself from all aspects of the review of this manuscript and all decisions made in regard to revisions/acceptance.
Figures

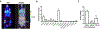

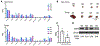
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

