Engineering a highly elastic bioadhesive for sealing soft and dynamic tissues
- PMID: 35148016
- PMCID: PMC9106862
- DOI: 10.1002/jbm.b.35012
Engineering a highly elastic bioadhesive for sealing soft and dynamic tissues
Erratum in
-
Correction to "Engineering a Highly Elastic Bioadhesive for Sealing Soft and Dynamic Tissues".J Biomed Mater Res B Appl Biomater. 2025 Aug;113(8):e35624. doi: 10.1002/jbm.b.35624. J Biomed Mater Res B Appl Biomater. 2025. PMID: 40728383 No abstract available.
Abstract
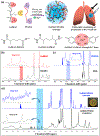
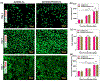
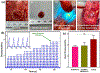
Injured tissues often require immediate closure to restore the normal functionality of the organ. In most cases, injuries are associated with trauma or various physical surgeries where different adhesive hydrogel materials are applied to close the wounds. However, these materials are typically toxic, have low elasticity, and lack strong adhesion especially to the wet tissues. In this study, a stretchable composite hydrogel consisting of gelatin methacrylol catechol (GelMAC) with ferric ions, and poly(ethylene glycol) diacrylate (PEGDA) was developed. The engineered material could adhere to the wet tissue surfaces through the chemical conjugation of catechol and methacrylate groups to the gelatin backbone. Moreover, the incorporation of PEGDA enhanced the elasticity of the bioadhesives. Our results showed that the physical properties and adhesion of the hydrogels could be tuned by changing the ratio of GelMAC/PEGDA. In addition, the in vitro toxicity tests confirmed the biocompatibility of the engineered bioadhesives. Finally, using an ex vivo lung incision model, we showed the potential application of the developed bioadhesives for sealing elastic tissues.
Keywords: adhesive; elastic; hydrogel; poly(ethylene glycol) diacrylate; sealant.
© 2022 Wiley Periodicals LLC.
Conflict of interest statement
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
Figures



References
-
- SAXENA AK. Synthetic biodegradable hydrogel (PleuraSeal) sealant for sealing of lung tissue after thoracoscopic resection. Journal of thoracic and cardiovascular surgery (Print) 2010;139(2):496–497. - PubMed
-
- Wain JC, Kaiser LR, Johnstone DW, Yang SC, Wright CD, Friedberg JS, Feins RH, Heitmiller RF, Mathisen DJ, Selwyn MR. Trial of a novel synthetic sealant in preventing air leaks after lung resection. The Annals of Thoracic Surgery 2001;71(5):1623–1629. - PubMed

