Deep Brain Stimulation for Arm Tremor: A Randomized Trial Comparing Two Targets
- PMID: 35148020
- PMCID: PMC9311445
- DOI: 10.1002/ana.26317
Deep Brain Stimulation for Arm Tremor: A Randomized Trial Comparing Two Targets
Abstract
Objective: Deep brain stimulation (DBS) of the thalamic ventral intermediate nucleus (VIM) effectively suppresses arm tremor. Uncontrolled studies suggest the posterior subthalamic area (PSA) may be superior. We compared the intra-individual efficacy of VIM- versus PSA-DBS on tremor suppression and arm function.
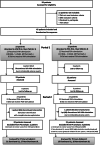
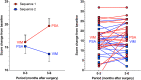
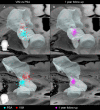
Methods: We performed a randomized, double-blind, crossover trial at Oslo University Hospital in patients (18-80 years) with isolated or combined action tremor affecting at least one arm. Four-contact DBS leads were implanted (bi- or unilaterally) with a trajectory to cover the VIM (upper two contacts) and PSA (lower two contacts). Patients were randomized (1:1 ratio) post-surgery to: Group 1, VIM-stimulation months 0-3 (period 1), then PSA-stimulation months 4-6 (period 2); Group 2, PSA-stimulation first, then VIM-stimulation. Primary endpoint was the difference in improvement from baseline to the end of the VIM- versus PSA-period in the sum of the dominant arm tremor scores of the Fahn-Tolosa-Marin Tremor Rating Scale (FTMTRS), items 5/6 + 10-14.
Results: Forty-five patients were randomized to Group 1 (n = 23) or 2 (n = 22). In the primary endpoint per-protocol analysis (mixed model, n = 40), mean difference in the sum FTMTRS score improvement for the dominant arm was -2.65 points (95% CI -4.33 to -0.97; p = 0.002). The difference in favour of PSA stimulation was highly significant in period 2, but not period 1.
Interpretation: Our randomized trial demonstrated that PSA stimulation provided superior tremor suppression compared with VIM stimulation. A period effect reducing tremor for up to three months in both groups was most likely attributed to a post-surgery stun effect. ANN NEUROL 2022;91:585-601.
© 2022 The Authors. Annals of Neurology published by Wiley Periodicals LLC on behalf of American Neurological Association.
Conflict of interest statement
Medtronic® and Boston Scientific® are both manufacturers of the DBS devices that have been used in this study. NK reports travel support from Medtronic to participate in a DBS course arranged by them. AEK reports travel support from Boston Scientific to attend a scientific meeting about DBS arranged by them. MMR reports consulting fees and honoraria for lectures from Boston Scientific and Medtronic, and from Boston Scientific also grants, board participation, and travel support for attending meetings, not directly related to the submitted work. JV reports grants and personal fees from Boston Scientific, grants and personal fees from Medtronic, and personal fees from Abbott St. Jude and Newronika (both manufacturers of DBS devices), not directly related to the submitted work. ED reports honoraria for lectures from AbbVie, Lobsor and Nordic Infucare (all manufacturers of dopaminergic medication pumps). IMS reports honoraria for lectures at scientific meetings and courses about DBS from Boston Scientific, arranged by them. None of the activities mentioned above were directly related to the submitted work. JR and AHP report no conflicts of interest.
Figures

References
-
- Deuschl G, Bain P, Brin M. Consensus statement of the Movement Disorder Society on tremor. Ad Hoc Scientific Committee. Mov Disord 1998;13:2–23. - PubMed
-
- Louis ED, Ferreira JJ. How common is the most common adult movement disorder? Update on the worldwide prevalence of essential tremor. Mov Disord 2010;25:534–541. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

