Versatile ginsenoside Rg3 liposomes inhibit tumor metastasis by capturing circulating tumor cells and destroying metastatic niches
- PMID: 35148178
- PMCID: PMC8836824
- DOI: 10.1126/sciadv.abj1262
Versatile ginsenoside Rg3 liposomes inhibit tumor metastasis by capturing circulating tumor cells and destroying metastatic niches
Abstract
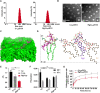
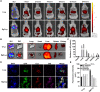
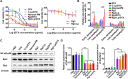
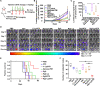
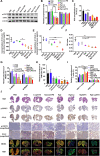
Limited circulating tumor cells (CTCs) capturing efficiency and lack of regulation capability on CTC-supportive metastatic niches (MNs) are two main obstacles hampering the clinical translation of conventional liposomes for the treatment of metastatic breast cancers. Traditional delivery strategies, such as ligand modification and immune modulator co-encapsulation for nanocarriers, are inefficient and laborious. Here, a multifunctional Rg3 liposome loading with docetaxel (Rg3-Lp/DTX) was developed, in which Rg3 was proved to intersperse in the phospholipid bilayer and exposed its glycosyl on the liposome surface. Therefore, it exhibited much higher CTC-capturing efficiency via interaction with glucose transporter 1 (Glut1) overexpressed on CTCs. After reaching the lungs with CTCs, Rg3 inhibited the formation of MNs by reversing the immunosuppressive microenvironment. Together, Rg3-Lp/DTX exhibited excellent metastasis inhibition capacity by CTC ("seeds") neutralization and MN ("soil") inhibition. The strategy has great clinical translation prospects for antimetastasis treatment with enhanced therapeutic efficacy and simple preparation process.
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

