Effect of trimetazidine on the functional capacity of ischemic heart disease patients not suitable for revascularization: Meta-analysis of randomized controlled trials
- PMID: 35148340
- PMCID: PMC8836318
- DOI: 10.1371/journal.pone.0263932
Effect of trimetazidine on the functional capacity of ischemic heart disease patients not suitable for revascularization: Meta-analysis of randomized controlled trials
Abstract
Objective: To explore the effect of adding trimetazidine to other anti-anginal drugs on the functional capacity of ischemic heart disease (IHD) patients not suitable for revascularization when compared to first-line antianginal drugs alone.
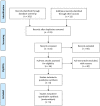
Methods: MEDLINE and EMBASE databases were searched for English-language peer-reviewed randomized controlled trials (RCTs) comparing trimetazidine with first-line antianginal drugs alone or with placebo in IHD patients not suitable for revascularization and were included in this review. Quality of studies were assessed using the Cochrane collaboration "risk of bias" tool.
Results: Six RCTs, three were crossover studies. A total of 312 participants were included in this review. Overall quality of studies was moderate. Two studies found improvement in the 6-minute walking test (6-MWT) [standardized mean differences (SMD) 1.75; 95% CI 1.35 to 2.14; p <0.001], and two trials found improvement in the Canadian cardiovascular society (CCS) grading of angina class (SMD -1.37; 95% CI -1.89 to -0.84) in the trimetazidine group. Three of the better-quality trials found no increase in total exercise duration (TED) (SMD 0.34; 95% CI -0.10 to 0.78; p < 0.13). Significant heterogeneity was identified among trials describing outcomes for the New York Heart Association (NYHA) functional classification and left ventricular ejection fraction (LVEF %).
Conclusion: Trimetazidine improve walking time and angina severity in IHD patients not suitable for revascularization. Due to the inconsistency of available evidence, RCTs targeting IHD patients with "no option" to undergo coronary revascularization is required to clarify this review question.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures


References
-
- Thadani U. The pursuit of optimum outcomes in stable angina. Am J Cardiovasc Drugs. 2003;3:11–2.
-
- Ferrari R, Ford I, Fox K, Marzilli M, Tendera M, Widimský P, et al.. A randomized, double-blind, placebo-controlled trial to assess the efficAcy and safety of Trimetazidine in patients with angina pectoris having been treated by percutaneous coronary intervention (ATPCI study): Rationale, design, and baseline characteristi. Am Heart J. 2019;210:98–107. doi: 10.1016/j.ahj.2018.12.015 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

