NCAM1 and GDF15 are biomarkers of Charcot-Marie-Tooth disease in patients and mice
- PMID: 35148379
- PMCID: PMC9679171
- DOI: 10.1093/brain/awac055
NCAM1 and GDF15 are biomarkers of Charcot-Marie-Tooth disease in patients and mice
Abstract
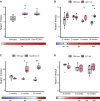
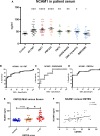
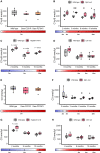
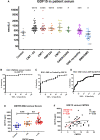
Molecular markers scalable for clinical use are critical for the development of effective treatments and the design of clinical trials. Here, we identify proteins in sera of patients and mouse models with Charcot-Marie-Tooth disease (CMT) with characteristics that make them suitable as biomarkers in clinical practice and therapeutic trials. We collected serum from mouse models of CMT1A (C61 het), CMT2D (GarsC201R, GarsP278KY), CMT1X (Gjb1-null), CMT2L (Hspb8K141N) and from CMT patients with genotypes including CMT1A (PMP22d), CMT2D (GARS), CMT2N (AARS) and other rare genetic forms of CMT. The severity of neuropathy in the patients was assessed by the CMT Neuropathy Examination Score (CMTES). We performed multitargeted proteomics on both sample sets to identify proteins elevated across multiple mouse models and CMT patients. Selected proteins and additional potential biomarkers, such as growth differentiation factor 15 (GDF15) and cell free mitochondrial DNA, were validated by ELISA and quantitative PCR, respectively. We propose that neural cell adhesion molecule 1 (NCAM1) is a candidate biomarker for CMT, as it was elevated in Gjb1-null, Hspb8K141N, GarsC201R and GarsP278KY mice as well as in patients with both demyelinating (CMT1A) and axonal (CMT2D, CMT2N) forms of CMT. We show that NCAM1 may reflect disease severity, demonstrated by a progressive increase in mouse models with time and a significant positive correlation with CMTES neuropathy severity in patients. The increase in NCAM1 may reflect muscle regeneration triggered by denervation, which could potentially track disease progression or the effect of treatments. We found that member proteins of the complement system were elevated in Gjb1-null and Hspb8K141N mouse models as well as in patients with both demyelinating and axonal CMT, indicating possible complement activation at the impaired nerve terminals. However, complement proteins did not correlate with the severity of neuropathy measured on the CMTES scale. Although the complement system does not seem to be a prognostic biomarker, we do show complement elevation to be a common disease feature of CMT, which may be of interest as a therapeutic target. We also identify serum GDF15 as a highly sensitive diagnostic biomarker, which was elevated in all CMT genotypes as well as in Hspb8K141N, Gjb1-null, GarsC201R and GarsP278KY mouse models. Although we cannot fully explain its origin, it may reflect increased stress response or metabolic disturbances in CMT. Further large and longitudinal patient studies should be performed to establish the value of these proteins as diagnostic and prognostic molecular biomarkers for CMT.
Keywords: Charcot-Marie-Tooth disease (CMT); biomarker; mouse models; serum; translational.
© The Author(s) 2022. Published by Oxford University Press on behalf of the Guarantors of Brain.
Figures




References
-
- Skre H. Genetic and clinical aspects of Charcot-Marie-Tooth’s disease. Clin Genet. 1974;6(2):98–118. - PubMed
-
- Barreto LCLS, Oliveira FS, Nunes PS, et al. Epidemiologic study of Charcot-Marie-Tooth disease: A systematic review. Neuroepidemiology. 2016;46(3):157–165. - PubMed
-
- Lupski JR, de Oca-Luna, Slaugenhaupt S, et al. DNA duplication associated with Charcot-Marie-Tooth disease type 1A. Cell 1991;66:219–232. - PubMed
-
- Timmerman V, Nelis E, Van Hul, et al. The peripheral myelin protein gene PMP-22 is contained within the Charcot-Marie-Tooth disease type 1A duplication. Nature genetics 1992;1:171–175. - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

