Tubulin tyrosination regulates synaptic function and is disrupted in Alzheimer's disease
- PMID: 35148384
- PMCID: PMC9337816
- DOI: 10.1093/brain/awab436
Tubulin tyrosination regulates synaptic function and is disrupted in Alzheimer's disease
Abstract
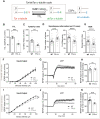
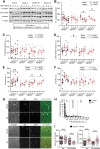
Microtubules play fundamental roles in the maintenance of neuronal processes and in synaptic function and plasticity. While dynamic microtubules are mainly composed of tyrosinated tubulin, long-lived microtubules contain detyrosinated tubulin, suggesting that the tubulin tyrosination/detyrosination cycle is a key player in the maintenance of microtubule dynamics and neuronal homeostasis, conditions that go awry in neurodegenerative diseases. In the tyrosination/detyrosination cycle, the C-terminal tyrosine of α-tubulin is removed by tubulin carboxypeptidases and re-added by tubulin tyrosine ligase (TTL). Here we show that TTL heterozygous mice exhibit decreased tyrosinated microtubules, reduced dendritic spine density and both synaptic plasticity and memory deficits. We further report decreased TTL expression in sporadic and familial Alzheimer's disease, and reduced microtubule dynamics in human neurons harbouring the familial APP-V717I mutation. Finally, we show that synapses visited by dynamic microtubules are more resistant to oligomeric amyloid-β peptide toxicity and that expression of TTL, by restoring microtubule entry into spines, suppresses the loss of synapses induced by amyloid-β peptide. Together, our results demonstrate that a balanced tyrosination/detyrosination tubulin cycle is necessary for the maintenance of synaptic plasticity, is protective against amyloid-β peptide-induced synaptic damage and that this balance is lost in Alzheimer's disease, providing evidence that defective tubulin retyrosination may contribute to circuit dysfunction during neurodegeneration in Alzheimer's disease.
Keywords: Alzheimer’s disease; dendritic spines; microtubule; neuron; tubulin.
© The Author(s) 2022. Published by Oxford University Press on behalf of the Guarantors of Brain.
Figures





References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

