The Challenges of Predicting Drug-Induced QTc Prolongation in Humans
- PMID: 35148401
- PMCID: PMC9041548
- DOI: 10.1093/toxsci/kfac013
The Challenges of Predicting Drug-Induced QTc Prolongation in Humans
Abstract
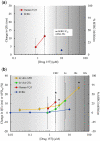
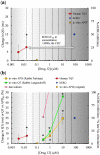
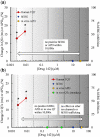
The content of this article derives from a Health and Environmental Sciences Institute (HESI) consortium with a focus to improve cardiac safety during drug development. A detailed literature review was conducted to evaluate the concordance between nonclinical repolarization assays and the clinical thorough QT (TQT) study. Food and Drug Administration and HESI developed a joint database of nonclinical and clinical data, and a retrospective analysis of 150 anonymized drug candidates was reviewed to compare the performance of 3 standard nonclinical assays with clinical TQT study findings as well as investigate mechanism(s) potentially responsible for apparent discrepancies identified. The nonclinical assays were functional (IKr) current block (Human ether-a-go-go related gene), action potential duration, and corrected QT interval in animals (in vivo corrected QT). Although these nonclinical assays demonstrated good specificity for predicting negative clinical QT prolongation, they had relatively poor sensitivity for predicting positive clinical QT prolongation. After review, 28 discordant TQT-positive drugs were identified. This article provides an overview of direct and indirect mechanisms responsible for QT prolongation and theoretical reasons for lack of concordance between clinical TQT studies and nonclinical assays. We examine 6 specific and discordant TQT-positive drugs as case examples. These were derived from the unique HESI/Food and Drug Administration database. We would like to emphasize some reasons for discordant data including, insufficient or inadequate nonclinical data, effects of the drug on other cardiac ion channels, and indirect and/or nonelectrophysiological effects of drugs, including altered heart rate. We also outline best practices that were developed based upon our evaluation.
Keywords: QT prolongation; Torsades de Pointes (TdP); discordance; hERG; pharmacokinetics; proarrhythmia; regulatory; sensitivity; specificity.
© The Author(s) 2022. Published by Oxford University Press on behalf of the Society of Toxicology.
Figures



References
-
- Abbott G. W., Sesti F., Splawski I., Buck M. E., Lehmann M. H., Timothy K. W., Keating M. T., Goldstein S. A. (1999). MiRP1 forms IKr potassium channels with HERG and is associated with cardiac arrhythmia. Cell 97, 175–187. - PubMed
-
- Abi-Gerges N., Philp K., Pollard C., Wakefield I., Hammond T. G., Valentin J. P. (2004). Sex differences in ventricular repolarization: From cardiac electrophysiology to Torsades de Pointes. Fund. Clin. Pharmacol. 18, 139–151. - PubMed
-
- Adelusi S. A., Salako L. A. (1982). Kinetics of the distribution and elimination of chloroquine in the rat. Gen. Pharmacol. 13, 433–437. - PubMed
-
- Anantharam A., Abbott G. W. (2005). Does hERG coassemble with a beta subunit? Evidence for roles of MinK and MiRP1. Novartis Found. Symp., 266, 100–112; discussion 112–107, 155–108. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

