Ferroptosis, as the most enriched programmed cell death process in glioma, induces immunosuppression and immunotherapy resistance
- PMID: 35148413
- PMCID: PMC9248406
- DOI: 10.1093/neuonc/noac033
Ferroptosis, as the most enriched programmed cell death process in glioma, induces immunosuppression and immunotherapy resistance
Abstract
Background: Immunosuppressive microenvironment is a major cause of immunotherapeutic resistance in glioma. In addition to secreting compounds, tumor cells under programmed cell death (PCD) processes release abundant mediators to modify the neighboring microenvironment. However, the complex relationship among PCD status, immunosuppressive microenvironment, and immunotherapy is still poorly understood.
Methods: Four independent glioma cohorts comprising 1,750 patients were enrolled for analysis. The relationships among PCD status, microenvironment cellular components, and biological phenotypes were fully explored. Tissues from our hospital and experiments in vitro and in vivo were used to confirm the role of ferroptosis in glioma.
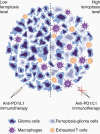
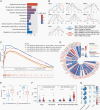
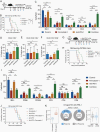
Results: Analyses to determine enriched PCD processes showed that ferroptosis was the main type of PCD in glioma. Enriched ferroptosis correlated with progressive malignancy, poor outcomes, and aggravated immunosuppression in glioblastoma (GBM) patients. Enhanced ferroptosis was shown to induce activation and infiltration of immune cells but attenuated antitumor cytotoxic killing. Tumor-associated macrophages (TAMs) were found to participate in ferroptosis-mediated immunosuppression. Preclinically, ferroptosis inhibition combined with Programmed Cell Death 1 (PD-1) and Programmed Cell Death Ligand-1 (PD-L1) blockade generated a synergistic therapeutic outcome in GBM murine models.
Conclusions: This work provides a molecular, clinical, and biological landscape of ferroptosis, suggesting a role of ferroptosis in glioma malignancy and a novel synergic immunotherapeutic strategy that combines immune checkpoint blockade treatment with ferroptosis inhibition.
Keywords: ICB; ferroptosis; immune microenvironment; immunotherapy; programmed cell death.
© The Author(s) 2022. Published by Oxford University Press on behalf of the Society for Neuro-Oncology. All rights reserved. For permissions, please e-mail: journals.permissions@oup.com.
Figures




References
-
- Tree A, Jones K, Hafeez S, et al. . Dose-limiting urinary toxicity with pembrolizumab combined with weekly hypofractionated radiation therapy in bladder cancer. Int J Radiat Oncol Biol Phys. 2018;101:1168–1171. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

