Soft X-ray tomography to map and quantify organelle interactions at the mesoscale
- PMID: 35148829
- PMCID: PMC9013509
- DOI: 10.1016/j.str.2022.01.006
Soft X-ray tomography to map and quantify organelle interactions at the mesoscale
Abstract
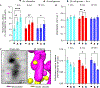
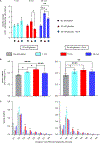
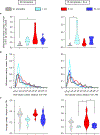
Inter-organelle interactions are a vital part of normal cellular function; however, these have proven difficult to quantify due to the range of scales encountered in cell biology and the throughput limitations of traditional imaging approaches. Here, we demonstrate that soft X-ray tomography (SXT) can be used to rapidly map ultrastructural reorganization and inter-organelle interactions in intact cells. SXT takes advantage of the naturally occurring, differential X-ray absorption of the carbon-rich compounds in each organelle. Specifically, we use SXT to map the spatiotemporal evolution of insulin vesicles and their co-localization and interaction with mitochondria in pancreatic β cells during insulin secretion and in response to different stimuli. We quantify changes in the morphology, biochemical composition, and relative position of mitochondria and insulin vesicles. These findings highlight the importance of a comprehensive and unbiased mapping at the mesoscale to characterize cell reorganization that would be difficult to detect with other existing methodologies.
Keywords: 3D cell mapping; mesoscale analysis; organelle interaction; pancreatic β cell; soft X-ray tomography.
Copyright © 2022 The Authors. Published by Elsevier Ltd.. All rights reserved.
Conflict of interest statement
Declaration of interests The authors declare no competing interests.
Figures



Similar articles
-
The use of soft X-ray tomography to explore mitochondrial structure and function.Mol Metab. 2022 Mar;57:101421. doi: 10.1016/j.molmet.2021.101421. Epub 2021 Dec 20. Mol Metab. 2022. PMID: 34942399 Free PMC article. Review.
-
Soft X-Ray Tomography Has Evolved into a Powerful Tool for Revealing Cell Structures.Annu Rev Anal Chem (Palo Alto Calif). 2025 May;18(1):427-446. doi: 10.1146/annurev-anchem-071124-093849. Epub 2025 Mar 3. Annu Rev Anal Chem (Palo Alto Calif). 2025. PMID: 40030074 Review.
-
Laboratory based correlative cryo-soft X-ray tomography and cryo-fluorescence microscopy.Methods Cell Biol. 2024;187:293-320. doi: 10.1016/bs.mcb.2024.02.033. Epub 2024 Mar 22. Methods Cell Biol. 2024. PMID: 38705628
-
An intensity-based post-processing tool for 3D instance segmentation of organelles in soft X-ray tomograms.PLoS One. 2022 Sep 1;17(9):e0269887. doi: 10.1371/journal.pone.0269887. eCollection 2022. PLoS One. 2022. PMID: 36048824 Free PMC article.
-
Soft X-ray tomograms provide a structural basis for whole-cell modeling.FASEB J. 2023 Jan;37(1):e22681. doi: 10.1096/fj.202200253R. FASEB J. 2023. PMID: 36519968 Free PMC article. Review.
Cited by
-
Subcellular Feature-Based Classification of α and β Cells Using Soft X-ray Tomography.Cells. 2024 May 18;13(10):869. doi: 10.3390/cells13100869. Cells. 2024. PMID: 38786091 Free PMC article.
-
Advanced Light Source Analytical Techniques for Exploring the Biological Behavior and Fate of Nanomedicines.ACS Cent Sci. 2022 Aug 24;8(8):1063-1080. doi: 10.1021/acscentsci.2c00680. Epub 2022 Aug 1. ACS Cent Sci. 2022. PMID: 36032763 Free PMC article. Review.
-
Montage electron tomography of vitrified specimens.J Struct Biol. 2022 Jun;214(2):107860. doi: 10.1016/j.jsb.2022.107860. Epub 2022 Apr 26. J Struct Biol. 2022. PMID: 35487464 Free PMC article.
-
Integrative structural modelling and visualisation of a cellular organelle.QRB Discov. 2022 Aug 9;3:e11. doi: 10.1017/qrd.2022.10. eCollection 2022. QRB Discov. 2022. PMID: 37529283 Free PMC article.
-
Automated 3D cytoplasm segmentation in soft X-ray tomography.iScience. 2024 Apr 29;27(6):109856. doi: 10.1016/j.isci.2024.109856. eCollection 2024 Jun 21. iScience. 2024. PMID: 38784019 Free PMC article.
References
-
- Attwood D. (2007). Soft x-rays and extreme ultraviolet radiation: principles and applications (Cambridge University Press; ).
-
- Bratanova-Tochkova TK, Cheng H, Daniel S, Gunawardana S, Liu Y-J, Mulvaney-Musa J, Schermerhorn T, Straub SG, Yajima H, and Sharp GWG (2002). Triggering and Augmentation Mechanisms, Granule Pools, and Biphasic Insulin Secretion. Diabetes 51, S83. - PubMed
-
- Buckley G, Gervinskas G, Taveneau C, Venugopal H, Whisstock JC, and de Marco A. (2019). Automated cryo-lamella preparation for high-throughput in-situ structural biology. bioRxiv. - PubMed

