Distinct EEG signatures differentiate unconsciousness and disconnection during anaesthesia and sleep
- PMID: 35148892
- PMCID: PMC9428919
- DOI: 10.1016/j.bja.2022.01.010
Distinct EEG signatures differentiate unconsciousness and disconnection during anaesthesia and sleep
Abstract
Background: How conscious experience becomes disconnected from the environment, or disappears, across arousal states is unknown. We sought to identify the neural correlates of sensory disconnection and unconsciousness using a novel serial awakening paradigm.
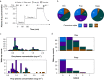
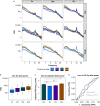
Methods: Volunteers were recruited for sedation with dexmedetomidine i.v., propofol i.v., or natural sleep with high-density EEG monitoring and serial awakenings to establish whether subjects were in states of disconnected consciousness or unconsciousness in the preceding 20 s. The primary outcome was classification of conscious states by occipital delta power (0.5-4 Hz). Secondary analyses included derivation (dexmedetomidine) and validation (sleep/propofol) studies of EEG signatures of conscious states.
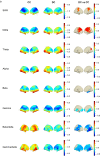
Results: Occipital delta power differentiated disconnected and unconscious states for dexmedetomidine (area under the curve [AUC] for receiver operating characteristic 0.605 [95% confidence interval {CI}: 0.516; 0.694]) but not for sleep/propofol (AUC 0.512 [95% CI: 0.380; 0.645]). Distinct source localised signatures of sensory disconnection (AUC 0.999 [95% CI: 0.9954; 1.0000]) and unconsciousness (AUC 0.972 [95% CI: 0.9507; 0.9879]) were identified using support vector machine classification of dexmedetomidine data. These findings generalised to sleep/propofol (validation data set: sensory disconnection [AUC 0.743 {95% CI: 0.6784; 0.8050}]) and unconsciousness (AUC 0.622 [95% CI: 0.5176; 0.7238]). We identified that sensory disconnection was associated with broad spatial and spectral changes. In contrast, unconsciousness was associated with focal decreases in activity in anterior and posterior cingulate cortices.
Conclusions: These findings may enable novel monitors of the anaesthetic state that can distinguish sensory disconnection and unconsciousness, and these may provide novel insights into the biology of arousal.
Clinical trial registration: NCT03284307.
Keywords: EEG; consciousness; dexmedetomidine; machine learning; propofol; sensory disconnection; sleep.
Copyright © 2022 British Journal of Anaesthesia. Published by Elsevier Ltd. All rights reserved.
Figures




Comment in
-
Consciousness and the outside world: is there anyone listening?Br J Anaesth. 2022 Jun;128(6):895-897. doi: 10.1016/j.bja.2022.02.001. Epub 2022 Mar 3. Br J Anaesth. 2022. PMID: 35249703
References
-
- Boly M., Sanders R.D., Mashour G.A., Laureys S. Consciousness and responsiveness: lessons from anaesthesia and the vegetative state. Curr Opin Anaesthesiol. 2013;26:444–449. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

