Human papillomavirus vaccine efficacy against invasive, HPV-positive cancers: population-based follow-up of a cluster-randomised trial
- PMID: 35149535
- PMCID: PMC8719207
- DOI: 10.1136/bmjopen-2021-050669
Human papillomavirus vaccine efficacy against invasive, HPV-positive cancers: population-based follow-up of a cluster-randomised trial
Abstract
Background: Human papillomavirus (HPV) vaccination protects against HPV, a necessary risk factor for cervical cancer. We now report results from population-based follow-up of randomised cohorts that vaccination provides HPV-type-specific protection against invasive cancer.
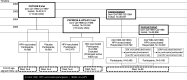
Methods: Individually and/or cluster randomised cohorts of HPV-vaccinated and non-vaccinated women were enrolled in 2002-2005. HPV vaccine cohorts comprised originally 16-17 year-old HPV 16/18-vaccinated PATRICIA (NCT00122681) and 012 trial (NCT00169494) participants (2465) and HPV6/11/16/18-vaccinated FUTURE II (NCT00092534) participants (866). Altogether, 3341 vaccines were followed by the Finnish Cancer Registry in the same way as 16 526 non-HPV-vaccinated controls. The control cohort stemmed from 15 665 originally 18-19 years-old women enrolled in 2003 (6499) or 2005 (9166) and 861 placebo recipients of the FUTURE II trial. The follow-up started 6 months after the clinical trials in 2007 and 2009 and ended in 2019. It was age aligned for the cohorts.
Findings: During a follow-up time of up to 11 years, we identified 17 HPV-positive invasive cancer cases (14 cervical cancers, 1 vaginal cancer, 1 vulvar cancer and 1 tongue cancer) in the non-HPV-vaccinated cohorts and no cases in the HPV-vaccinated cohorts. HPV typing of diagnostic tumour blocks found HPV16 in nine cervical cancer cases, HPV18, HPV33 and HPV52 each in two cases and HPV45 in one cervical cancer case. The vaginal, vulvar and tongue cancer cases were, respectively, positive for HPV16, HPV52/66 and HPV213. Intention-to-treat vaccine efficacy against all HPV-positive cancers was 100% (95% CI 2 to 100, p<0.05).
Interpretation: Vaccination is effective against invasive HPV-positive cancer.
Trial registration number: NCT00122681, Post-results; NCT00169494, Post-results; NCT00092534, Post-results.
Keywords: epidemiology; gynaecological oncology; preventive medicine; public health; sexual medicine.
© Author(s) (or their employer(s)) 2021. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: We declare that ML, DA, JD and JPaa have received grants from Merck & Co. Inc. and/or from the GSK group of companies through their respective employers. GSK Biologicals SA was provided the opportunity to review this manuscript but the authors are solely responsible for final content and interpretation.
Figures
References
-
- Lehtinen M, Lagheden C, Luostarinen T, et al. . Ten-year follow-up of human papillomavirus vaccine efficacy against the most stringent cervical neoplasia end-point-registry-based follow-up of three cohorts from randomized trials. BMJ Open 2017;7:e015867. 10.1136/bmjopen-2017-015867 - DOI - PMC - PubMed
-
- Kjaer SK, Nygård M, Sundström K, et al. . Final analysis of a 14-year long-term follow-up study of the effectiveness and immunogenicity of the quadrivalent human papillomavirus vaccine in women from four Nordic countries. EClinicalMedicine 2020;23:100401. 10.1016/j.eclinm.2020.100401 - DOI - PMC - PubMed
