Targeting stem-loop 1 of the SARS-CoV-2 5' UTR to suppress viral translation and Nsp1 evasion
- PMID: 35149555
- PMCID: PMC8892331
- DOI: 10.1073/pnas.2117198119
Targeting stem-loop 1 of the SARS-CoV-2 5' UTR to suppress viral translation and Nsp1 evasion
Abstract
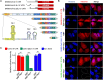
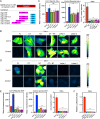
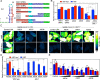
SARS-CoV-2 is a highly pathogenic virus that evades antiviral immunity by interfering with host protein synthesis, mRNA stability, and protein trafficking. The SARS-CoV-2 nonstructural protein 1 (Nsp1) uses its C-terminal domain to block the messenger RNA (mRNA) entry channel of the 40S ribosome to inhibit host protein synthesis. However, how SARS-CoV-2 circumvents Nsp1-mediated suppression for viral protein synthesis and if the mechanism can be targeted therapeutically remain unclear. Here, we show that N- and C-terminal domains of Nsp1 coordinate to drive a tuned ratio of viral to host translation, likely to maintain a certain level of host fitness while maximizing replication. We reveal that the stem-loop 1 (SL1) region of the SARS-CoV-2 5' untranslated region (5' UTR) is necessary and sufficient to evade Nsp1-mediated translational suppression. Targeting SL1 with locked nucleic acid antisense oligonucleotides inhibits viral translation and makes SARS-CoV-2 5' UTR vulnerable to Nsp1 suppression, hindering viral replication in vitro at a nanomolar concentration, as well as providing protection against SARS-CoV-2-induced lethality in transgenic mice expressing human ACE2. Thus, SL1 allows Nsp1 to switch infected cells from host to SARS-CoV-2 translation, presenting a therapeutic target against COVID-19 that is conserved among immune-evasive variants. This unique strategy of unleashing a virus' own virulence mechanism against itself could force a critical trade-off between drug resistance and pathogenicity.
Keywords: SARS-CoV-2; therapeutic; translation.
Copyright © 2022 the Author(s). Published by PNAS.
Conflict of interest statement
The authors declare no competing interest.
Figures



Update of
-
SARS-CoV-2 Nsp1 suppresses host but not viral translation through a bipartite mechanism.bioRxiv [Preprint]. 2020 Sep 18:2020.09.18.302901. doi: 10.1101/2020.09.18.302901. bioRxiv. 2020. Update in: Proc Natl Acad Sci U S A. 2022 Mar 1;119(9):e2117198119. doi: 10.1073/pnas.2117198119. PMID: 32995777 Free PMC article. Updated. Preprint.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

