Treatment Outcome of Wet Age-Related Macular Degeneration Management in Thailand: A Retrospective Real-World Study (TOWER Study)
- PMID: 35149964
- PMCID: PMC8927559
- DOI: 10.1007/s40123-022-00471-5
Treatment Outcome of Wet Age-Related Macular Degeneration Management in Thailand: A Retrospective Real-World Study (TOWER Study)
Abstract
Introduction: To present real-world outcomes of neovascular age-related macular degeneration (nAMD) management in Thailand.
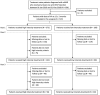
Methods: This multicenter retrospective study reviewed medical records of naive nAMD patients diagnosed from 1 January 2016 until 31 December 2018. The patients received at least one intravitreal anti-vascular endothelial growth factor (VEGF) treatment and had captured visual acuity (VA) at baseline and at month 12. Treatment outcomes were assessed at month 12, 24, and 36. The primary outcome was a mean change in VA from baseline to month 12.
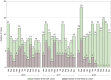
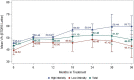
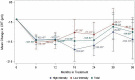
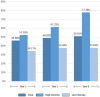
Results: Five hundred seventy-two (572) eyes were included in this study and of these eyes, 222 and 96 had 2- and 3-year follow-up periods, respectively. At month 12, the mean improvement of VA (ETDRS letter) was six letters (P < 0.0001), and central retinal thickness (CRT) decreased on average by 104 microns (P < 0.0001). However, visual improvement by 0.1 letters at month 36 did not show statistical significance. The presence of fluid was found in approximately half of patients throughout the study period (45.98%, 48.85%, and 50.91% at month 12, 24, and 36, respectively). Mean number of injections (SD) was 6.06 (3.00), 3.44 (2.94), and 2.71 (3.07) for years 1, 2, and 3, respectively. The mean number of visits (SD) in year 1 was 9.01 (2.60) and declined to 5.67 (2.69) in year 2 and 4.93 (2.49) in year 3. Patients who had an average injection interval of ≤ 8 weeks were 74.46% in year 1, 51.28% in year 2, and 45.24 in year 3; 35.31% of patients were lost to follow-up.
Conclusions: This analysis reflects real-world nAMD management with significant improvement of outcomes. At the same time, the study reveals unmet needs in anti-VEGF therapy in nAMD including persistent disease activities, inadequacy of available treatment, and lack of treatment adherence leading to visual deterioration in the long-term.
Keywords: Aflibercept; Anti-VEGF; Bevacizumab; Neovascular age-related macular degeneration; Ranibizumab; Real-world study; nAMD.
© 2022. The Author(s).
Figures


References
-
- Blindness GBD, Vision Impairment C, Vision Loss Expert Group of the Global Burden of Disease S Causes of blindness and vision impairment in 2020 and trends over 30 years, and prevalence of avoidable blindness in relation to VISION 2020: the Right to Sight: an analysis for the Global Burden of Disease Study. Lancet Glob Health. 2021;9(2):e144–e160. doi: 10.1016/S2214-109X(20)30489-7. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

