The clinical pharmacology and potential therapeutic applications of 5-methoxy-N,N-dimethyltryptamine (5-MeO-DMT)
- PMID: 35149998
- PMCID: PMC9314805
- DOI: 10.1111/jnc.15587
The clinical pharmacology and potential therapeutic applications of 5-methoxy-N,N-dimethyltryptamine (5-MeO-DMT)
Abstract
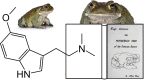
5-methoxy-N,N-dimethyltryptamine (5-MeO-DMT) is a naturally occurring tryptamine that primarily acts as an agonist at the 5-HT1A and 5-HT2A receptors, whereby affinity for the 5-HT1A subtype is highest. Subjective effects following 5-MeO-DMT administration include distortions in auditory and time perception, amplification of emotional states, and feelings of ego dissolution that usually are short-lasting, depending on the route of administration. Individual dose escalation of 5-MeO-DMT reliably induces a "peak" experience, a state thought to be a core predictor of the therapeutic efficacy of psychedelics. Observational studies and surveys have suggested that single exposure to 5-MeO-DMT can cause rapid and sustained reductions in symptoms of depression, anxiety, and stress. 5-MeO-DMT also stimulates neuroendocrine function, immunoregulation, and anti-inflammatory processes, which may contribute to changes in mental health outcomes. To date, only one clinical trial has been published on 5-MeO-DMT, demonstrating the safety of vaporized dosing up to 18 mg. Importantly, the rapid onset and short duration of the 5-MeO-DMT experience may render it more suitable for individual dose-finding strategies compared with longer-acting psychedelics. A range of biotech companies has shown an interest in the development of 5-MeO-DMT formulations for a range of medical indications, most notably depression. Commercial development will therefore be the most important resource for bringing 5-MeO-DMT to the clinic. However, fundamental research will also be needed to increase understanding of the neurophysiological and neural mechanisms that contribute to the potential clinical effects of 5-MeO-DMT and its sustainability and dissemination over time. Such studies are less likely to be conducted as part of drug development programs and are more likely to rely on independent, academic initiatives.
Keywords: 5-MeO-DMT; clinical development; mental health; neuroinflammation.
© 2022 The Authors. Journal of Neurochemistry published by John Wiley & Sons Ltd on behalf of International Society for Neurochemistry.
Conflict of interest statement
JTR and JGR are scientific consultants to GH Research. MVU is a scientific consultant to Entheon. AKD is supported by private philanthropic funding from Tim Ferriss, Matt Mullenweg, Craig Nerenberg, Blake Mycoskie, and the Steven and Alexandra Cohen Foundation. AKD is also supported by the Center for Psychedelic Drug Research and Education, funded by anonymous private donors. The funding sources had no role in the study, data analysis, interpretation, or communication of findings.
Figures

References
-
- Agurell, S. , Holmstedt, B. , Lindgren, J. E. , & Schultes, R. E. (1969). Alkaloids in certain species of Virola and other South American plants of ethnopharmacologic interest. Acta Chemica Scandinavica, 23, 903–916. - PubMed
-
- AlvariusPharmaceuticals (2021). https://alvarius.com/.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

