Contribution of common and rare genetic variants in CEP72 on vincristine-induced peripheral neuropathy in brain tumour patients
- PMID: 35150001
- PMCID: PMC9313903
- DOI: 10.1111/bcp.15267
Contribution of common and rare genetic variants in CEP72 on vincristine-induced peripheral neuropathy in brain tumour patients
Abstract
Aims: Studies implicated a role for a genetic variant in CEP72 in vincristine-induced peripheral neuropathy. This study aims to evaluate this association in a cohort of brain tumour patients, to perform a cross-disease meta-analysis and explore the protein-coding region of CEP72.
Methods: In total, 104 vincristine-treated brain tumour patients were genotyped for CEP72 rs924607, and sequenced for the protein-coding region. Data regarding patient and treatment characteristics, and peripheral neuropathy, were collected. Logistic regression and meta-analysis were performed for rs924607 replication. A weighted burden analysis was applied to evaluate impact of overall genetic variation in CEP72.
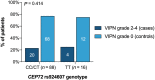
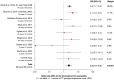
Results: Analysis of 24 cases and 80 controls did not show a significant association between CEP72 rs924607 and neuropathy (odds ratio, OR [95% confidence interval, CI] 2.076 [0.359-11.989], P = .414). When combined with 8 cohorts (1095 cancer patients), a significant increase in risk for neuropathy was found for patients with a TT genotype (OR [95% CI] 2.15 [1.35-3.43], P = .001). Additionally, a missense variant (rs12522955) was significantly associated (OR [95% CI] 2.3 [1.2-4.4], P = .041) and patients with severe neuropathy carried more impactful variants in CEP72 coding regions (P = .039).
Conclusion: The association of CEP72 rs924607 in vincristine-induced neuropathy was not confirmed in a cohort of brain tumour patients, but did contribute to its suggested effect when combined in a cross-disease meta-analysis. The importance of other genetic variations in CEP72 on vincristine-induced neuropathy was demonstrated. This study contributes to evidence of the importance of genetic variants in CEP72 in development of vincristine-induced toxicity, and provides guidance for future prospective studies.
Keywords: CEP72; brain tumours; neuropathy; pharmacogenetics; vincristine.
© 2022 The Authors. British Journal of Clinical Pharmacology published by John Wiley & Sons Ltd on behalf of British Pharmacological Society.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

