Effects of Vivifrail multicomponent intervention on functional capacity: a multicentre, randomized controlled trial
- PMID: 35150086
- PMCID: PMC8977963
- DOI: 10.1002/jcsm.12925
Effects of Vivifrail multicomponent intervention on functional capacity: a multicentre, randomized controlled trial
Abstract
Background: Physical exercise is an effective strategy for preserving functional capacity and improving the symptoms of frailty in older adults. In addition to functional gains, exercise is considered to be a cornerstone for enhancing cognitive function in frail older adults with cognitive impairment and dementia. We assessed the effects of the Vivifrail exercise intervention for functional capacity, cognition, and well-being status in community-dwelling older adults.
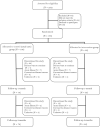
Methods: In a multicentre randomized controlled trial conducted in three tertiary hospitals in Spain, a total of 188 older patients with mild cognitive impairment or mild dementia (aged >75 years) were randomly assigned to an exercise intervention (n = 88) or a usual-care, control (n = 100) group. The intervention was based on the Vivifrail tailored multicomponent exercise programme, which included resistance, balance, flexibility (3 days/week), and gait-retraining exercises (5 days/week) and was performed for three consecutive months (http://vivifrail.com). The usual-care group received habitual outpatient care. The main endpoint was change in functional capacity from baseline to 1 and 3 months, assessed with the Short Physical Performance Battery (SPPB). Secondary endpoints were changes in cognitive function and handgrip strength after 1 and 3 months, and well-being status, falls, hospital admission rate, visits to the emergency department, and mortality after 3 months.
Results: The Vivifrail exercise programme provided significant benefits in functional capacity over usual-care. The mean adherence to the exercise sessions was 79% in the first month and 68% in the following 2 months. The intervention group showed a mean increase (over the control group) of 0.86 points on the SPPB scale (95% confidence interval [CI] 0.32, 1.41 points; P < 0.01) after 1 month of intervention and 1.40 points (95% CI 0.82, 1.98 points; P < 0.001) after 3 months. Participants in the usual-care group showed no significant benefit in functional capacity (mean change of -0.17 points [95% CI -0.54, 0.19 points] after 1 month and -0.33 points [95% CI -0.70, 0.04 points] after 3 months), whereas the exercise intervention reversed this trend (0.69 points [95% CI 0.29, 1.09 points] after 1 month and 1.07 points [95% CI 0.63, 1.51 points] after 3 months). Exercise group also obtained significant benefits in cognitive function, muscle function, and depression after 3 months over control group (P < 0.05). No between-group differences were obtained in other secondary endpoints (P > 0.05).
Conclusions: The Vivifrail exercise training programme is an effective and safe therapy for improving functional capacity in community-dwelling frail/prefrail older patients with mild cognitive impairment or mild dementia and also seems to have beneficial effect on cognition, muscle function, and mood status.
Trial registration: ClinicalTrials.gov NCT03657940.
Keywords: Falls; Frailty; Functional capacity; Multicomponent exercise programme.
© 2022 The Authors. Journal of Cachexia, Sarcopenia and Muscle published by John Wiley & Sons Ltd on behalf of Society on Sarcopenia, Cachexia and Wasting Disorders.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Comment in
-
Comment on 'Effects of Vivifrail multicomponent intervention on functional capacity: A multicentre randomized controlled trial' by Casas-Herrero et al.J Cachexia Sarcopenia Muscle. 2023 Oct;14(5):2454. doi: 10.1002/jcsm.13296. Epub 2023 Aug 30. J Cachexia Sarcopenia Muscle. 2023. PMID: 37649321 Free PMC article. No abstract available.
-
Comment on "Effects of Vivifrail multicomponent intervention on functional capacity" by Casas-Herrero et al.-The authors reply.J Cachexia Sarcopenia Muscle. 2024 Feb;15(1):457-460. doi: 10.1002/jcsm.13387. Epub 2023 Dec 3. J Cachexia Sarcopenia Muscle. 2024. PMID: 38044035 Free PMC article. No abstract available.
References
-
- Hoogendijk EO, Afilalo J, Ensrud KE, Kowal P, Onder G, Fried LP. Frailty: implications for clinical practice and public health. Lancet Lond Engl 2019;394:1365–1375. - PubMed
-
- Ward DD, Wallace LMK, Rockwood K. Frailty and risk of dementia in mild cognitive impairment subtypes. Ann Neurol. Published online March 11 2021;89:1221–1225. - PubMed
-
- Robertson DA, Savva GM, Kenny RA. Frailty and cognitive impairment‐‐a review of the evidence and causal mechanisms. Ageing Res Rev 2013;12:840–851. - PubMed
-
- Rodriguez‐Mañas L, Fried LP. Frailty in the clinical scenario. Lancet Lond Engl 2015;385:e7–e9. - PubMed
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

