Ocular Nanomedicine
- PMID: 35150092
- PMCID: PMC9130902
- DOI: 10.1002/advs.202003699
Ocular Nanomedicine
Abstract
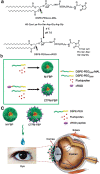
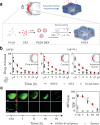
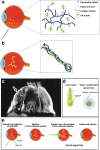
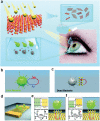
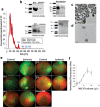
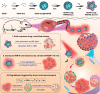
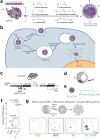
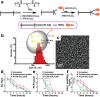
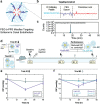
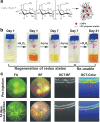
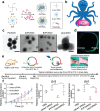
Intrinsic shortcomings associated with conventional therapeutic strategies often compromise treatment efficacy in clinical ophthalmology, prompting the rapid development of versatile alternatives for satisfactory diagnostics and therapeutics. Given advances in material science, nanochemistry, and nanobiotechnology, a broad spectrum of functional nanosystems has been explored to satisfy the extensive requirements of ophthalmologic applications. In the present review, the recent progress in nanosystems, both conventional and emerging nanomaterials in ophthalmology from state-of-the-art studies, are comprehensively examined and the role of their fundamental physicochemical properties in bioavailability, tissue penetration, biodistribution, and elimination after interacting with the ophthalmologic microenvironment emphasized. Furthermore, along with the development of surface engineering of nanomaterials, emerging theranostic methodologies are promoted as potential alternatives for multipurpose ocular applications, such as emerging biomimetic ophthalmology (e.g., smart electrochemical eye), thus provoking a holistic review of "ocular nanomedicine." By affording insight into challenges encountered by ocular nanomedicine and further highlighting the direction of future studies, this review provides an incentive for enriching ocular nanomedicine-based fundamental research and future clinical translation.
Keywords: diagnostics; nanomedicine; ocular; ophthalmology; therapeutics.
© 2022 The Authors. Advanced Science published by Wiley-VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures






References
-
- Ma Y., Bao J., Zhang Y., Li Z., Zhou X., Wan C., Huang L., Zhao Y., Han G., Xue T., Cell 2019, 177, 243. - PubMed
-
- a) Wang X., Lou N., Eberhardt A., Yang Y., Kusk P., Xu Q., Förstera B., Peng S., Shi M., Ladrón‐de‐Guevara A., Delle C., Sigurdsson B., Xavier A. L. R., Ertürk A., Libby R. T., Chen L., Thrane A. S., Nedergaard M., Sci. Transl. Med. 2020, 12, eaaw3210; - PMC - PubMed
- b) Roudnicky F., Zhang J. D., Kim B. K., Pandya N. J., Lan Y., Sach‐Peltason L., Ragelle H., Strassburger P., Gruener S., Lazendic M., Uhles S., Revelant F., Eidam O., Sturm G., Kueppers V., Christensen K., Goldstein L. D., Tzouros M., Banfai B., Modrusan Z., Graf M., Patsch C., Burcin M., Meyer C. A., Westenskow P. D., Cowan C. A., Proc. Natl. Acad. Sci. USA 2020, 117, 19854. - PMC - PubMed
-
- Burton M. J., Ramke J., Marques A. P., Bourne R. R. A., Congdon N., Jones I., Ah Tong B. A. M., Arunga S., Bachani D., Bascaran C., Bastawrous A., Blanchet K., Braithwaite T., Buchan J. C., Cairns J., Cama A., Chagunda M., Chuluunkhuu C., Cooper A., Crofts‐Lawrence J., Dean W. H., Denniston A. K., Ehrlich J. R., Emerson P. M., Evans J. R., Frick K. D., Friedman D. S., Furtado J. M., Gichangi M. M., Gichuhi S., et al., Lancet Glob. Health. 2021, 9, e489. - PubMed
-
- Ren N., Sun R., Xia K., Zhang Q., Li W., Wang F., Zhang X., Ge Z., Wang L., Fan C., Zhu Y., ACS Appl. Mater. Interfaces 2019, 11, 26704. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
