RadD is a RecA-dependent accessory protein that accelerates DNA strand exchange
- PMID: 35150260
- PMCID: PMC8887467
- DOI: 10.1093/nar/gkac041
RadD is a RecA-dependent accessory protein that accelerates DNA strand exchange
Abstract
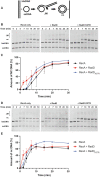
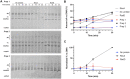
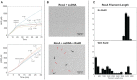
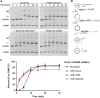
In rapidly growing cells, with recombinational DNA repair required often and a new replication fork passing every 20 min, the pace of RecA-mediated DNA strand exchange is potentially much too slow for bacterial DNA metabolism. The enigmatic RadD protein, a putative SF2 family helicase, exhibits no independent helicase activity on branched DNAs. Instead, RadD greatly accelerates RecA-mediated DNA strand exchange, functioning only when RecA protein is present. The RadD reaction requires the RadD ATPase activity, does not require an interaction with SSB, and may disassemble RecA filaments as it functions. We present RadD as a new class of enzyme, an accessory protein that accelerates DNA strand exchange, possibly with a helicase-like action, in a reaction that is entirely RecA-dependent. RadD is thus a DNA strand exchange (recombination) synergist whose primary function is to coordinate closely with and accelerate the DNA strand exchange reactions promoted by the RecA recombinase. Multiple observations indicate a uniquely close coordination of RadD with RecA function.
© The Author(s) 2022. Published by Oxford University Press on behalf of Nucleic Acids Research.
Figures

References
-
- Courcelle J., Belle J.J., Courcelle C.T.. When replication travels on damaged templates: bumps and blocks in the road. Res. Microbiol. 2004; 155:231–237. - PubMed
-
- Cox M.M., Goodman M.F., Kreuzer K.N., Sherratt D.J., Sandler S.J., Marians K.J.. The importance of repairing stalled replication forks. Nature. 2000; 404:37–41. - PubMed
-
- Kowalczykowski S.C. Initiation of genetic recombination and recombination-dependent replication. Trends Biochem. Sci. 2000; 25:156–165. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

