High-titre methylene blue-treated convalescent plasma as an early treatment for outpatients with COVID-19: a randomised, placebo-controlled trial
- PMID: 35150610
- PMCID: PMC8828369
- DOI: 10.1016/S2213-2600(21)00545-2
High-titre methylene blue-treated convalescent plasma as an early treatment for outpatients with COVID-19: a randomised, placebo-controlled trial
Abstract
Background: Convalescent plasma has been proposed as an early treatment to interrupt the progression of early COVID-19 to severe disease, but there is little definitive evidence. We aimed to assess whether early treatment with convalescent plasma reduces the risk of hospitalisation and reduces SARS-CoV-2 viral load among outpatients with COVID-19.
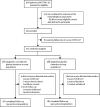
Methods: We did a multicentre, double-blind, randomised, placebo-controlled trial in four health-care centres in Catalonia, Spain. Adult outpatients aged 50 years or older with the onset of mild COVID-19 symptoms 7 days or less before randomisation were eligible for enrolment. Participants were randomly assigned (1:1) to receive one intravenous infusion of either 250-300 mL of ABO-compatible high anti-SARS-CoV-2 IgG titres (EUROIMMUN ratio ≥6) methylene blue-treated convalescent plasma (experimental group) or 250 mL of sterile 0·9% saline solution (control). Randomisation was done with the use of a central web-based system with concealment of the trial group assignment and no stratification. To preserve masking, we used opaque tubular bags that covered the investigational product and the infusion catheter. The coprimary endpoints were the incidence of hospitalisation within 28 days from baseline and the mean change in viral load (in log10 copies per mL) in nasopharyngeal swabs from baseline to day 7. The trial was stopped early following a data safety monitoring board recommendation because more than 85% of the target population had received a COVID-19 vaccine. Primary efficacy analyses were done in the intention-to-treat population, safety was assessed in all patients who received the investigational product. This study is registered with ClinicalTrials.gov, NCT04621123.
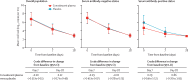
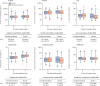
Findings: Between Nov 10, 2020, and July 28, 2021, we assessed 909 patients with confirmed COVID-19 for inclusion in the trial, 376 of whom were eligible and were randomly assigned to treatment (convalescent plasma n=188 [serum antibody-negative n=160]; placebo n=188 [serum antibody-negative n=166]). Median age was 56 years (IQR 52-62) and the mean symptom duration was 4·4 days (SD 1·4) before random assignment. In the intention-to-treat population, hospitalisation within 28 days from baseline occurred in 22 (12%) participants who received convalescent plasma versus 21 (11%) who received placebo (relative risk 1·05 [95% CI 0·78 to 1·41]). The mean change in viral load from baseline to day 7 was -2·41 log10 copies per mL (SD 1·32) with convalescent plasma and -2·32 log10 copies per mL (1·43) with placebo (crude difference -0·10 log10 copies per mL [95% CI -0·35 to 0·15]). One participant with mild COVID-19 developed a thromboembolic event 7 days after convalescent plasma infusion, which was reported as a serious adverse event possibly related to COVID-19 or to the experimental intervention.
Interpretation: Methylene blue-treated convalescent plasma did not prevent progression from mild to severe illness and did not reduce viral load in outpatients with COVID-19. Therefore, formal recommendations to support the use of convalescent plasma in outpatients with COVID-19 cannot be concluded.
Funding: Grifols, Crowdfunding campaign YoMeCorono.
Copyright © 2022 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Declaration of interests We declare no competing interests.
Figures
Comment in
-
Convalescent plasma in outpatients with COVID-19.Lancet Respir Med. 2022 Mar;10(3):226-228. doi: 10.1016/S2213-2600(22)00050-9. Epub 2022 Feb 9. Lancet Respir Med. 2022. PMID: 35150608 Free PMC article. No abstract available.
References
-
- US Food and Drug Administration Coronavirus (COVID-19) update: FDA authorizes monoclonal antibodies for treatment of COVID-19. https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19...
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

