Matrix remodeling controls a nuclear lamin A/C-emerin network that directs Wnt-regulated stem cell fate
- PMID: 35150612
- PMCID: PMC8891068
- DOI: 10.1016/j.devcel.2022.01.015
Matrix remodeling controls a nuclear lamin A/C-emerin network that directs Wnt-regulated stem cell fate
Abstract
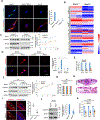
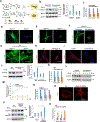
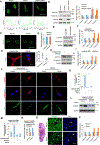
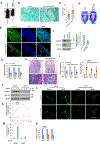
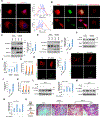
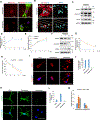
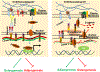
Skeletal stem cells (SSCs) reside within a three-dimensional extracellular matrix (ECM) compartment and differentiate into multiple cell lineages, thereby controlling tissue maintenance and regeneration. Within this environment, SSCs can proteolytically remodel the surrounding ECM in response to growth factors that direct lineage commitment via undefined mechanisms. Here, we report that Mmp14-dependent ECM remodeling coordinates canonical Wnt signaling and guides stem cell fate by triggering an integrin-activated reorganization of the SCC cytoskeleton that controls nuclear lamin A/C levels via the linker of nucleoskeleton and cytoskeleton (LINC) complexes. In turn, SSC lamin A/C levels dictate the localization of emerin, an inner nuclear membrane protein whose ability to regulate β-catenin activity modulates Wnt signaling while directing lineage commitment in vitro and in vivo. These findings define a previously undescribed axis wherein SSCs use Mmp14-dependent ECM remodeling to control cytoskeletal and nucleoskeletal organization, thereby governing Wnt-dependent stem cell fate decisions.
Keywords: Mmp14; Wnt; adipogenesis; beta-catenin; collagen; emerin; extracellular matrix; lamin; matrix metalloproteinase; osteogenesis; stem cells.
Copyright © 2022 Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests The authors declare no competing interests.
Figures
References
-
- Baron R, and Kneissel M (2013). WNT signaling in bone homeostasis and disease: from human mutations to treatments. Nat Med 19, 179–192. - PubMed
-
- Bermeo S, Vidal C, Zhou H, and Duque G (2015). Lamin A/C acts as an essential factor in mesenchymal stem cell differentiation through the regulation of the dynamics of the Wnt/beta-catenin pathway. J Cell Biochem 116, 2344–2353. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

