Single-virus assay reveals membrane determinants and mechanistic features of Sendai virus binding
- PMID: 35150620
- PMCID: PMC8943810
- DOI: 10.1016/j.bpj.2022.02.011
Single-virus assay reveals membrane determinants and mechanistic features of Sendai virus binding
Abstract
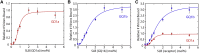
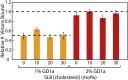
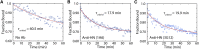
Sendai virus (SeV, formally murine respirovirus) is a membrane-enveloped, negative-sense RNA virus in the Paramyxoviridae family and is closely related to human parainfluenza viruses. SeV has long been utilized as a model paramyxovirus and has recently gained attention as a viral vector candidate for both laboratory and clinical applications. To infect host cells, SeV must first bind to sialic acid glycolipid or glycoprotein receptors on the host cell surface via its hemagglutinin-neuraminidase (HN) protein. Receptor binding induces a conformational change in HN, which allosterically triggers the viral fusion (F) protein to catalyze membrane fusion. While it is known that SeV binds to α2,3-linked sialic acid receptors, and there has been some study into the chemical requirements of those receptors, key mechanistic features of SeV binding remain unknown, in part because traditional approaches often convolve binding and fusion. Here, we develop and employ a fluorescence microscopy-based assay to observe SeV binding to supported lipid bilayers (SLBs) at the single-particle level, which easily disentangles binding from fusion. Using this assay, we investigate mechanistic questions of SeV binding. We identify chemical structural features of ganglioside receptors that influence viral binding and demonstrate that binding is cooperative with respect to receptor density. We measure the characteristic decay time of unbinding and provide evidence supporting a "rolling" mechanism of viral mobility following receptor binding. We also study the dependence of binding on target cholesterol concentration. Interestingly, we find that although SeV binding shows striking parallels in cooperative binding with a prior report of Influenza A virus, it does not demonstrate a similar sensitivity to cholesterol concentration and receptor nanocluster formation.
Copyright © 2022 Biophysical Society. Published by Elsevier Inc. All rights reserved.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

