The tocopherol transfer protein mediates vitamin E trafficking between cerebellar astrocytes and neurons
- PMID: 35150738
- PMCID: PMC8913317
- DOI: 10.1016/j.jbc.2022.101712
The tocopherol transfer protein mediates vitamin E trafficking between cerebellar astrocytes and neurons
Abstract
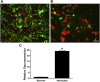
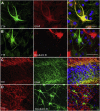
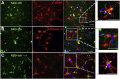
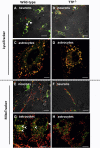
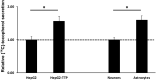
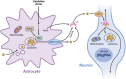
Alpha-tocopherol (vitamin E) is an essential nutrient that functions as a major lipid-soluble antioxidant in humans. The alpha-tocopherol transfer protein (TTP) binds α-tocopherol with high affinity and selectivity and regulates whole-body distribution of the vitamin. Heritable mutations in the TTPA gene result in familial vitamin E deficiency, elevated indices of oxidative stress, and progressive neurodegeneration that manifest primarily in spinocerebellar ataxia. Although the essential role of vitamin E in neurological health has been recognized for over 50 years, the mechanisms by which this essential nutrient is transported in the central nervous system are poorly understood. Here we found that, in the murine cerebellum, TTP is selectively expressed in glial fibrillary acidic protein-positive astrocytes, where it facilitates efflux of vitamin E to neighboring neurons. We also show that induction of oxidative stress enhances the transcription of the TtpA gene in cultured cerebellar astrocytes. Furthermore, secretion of vitamin E from astrocytes is mediated by an ABC-type transporter, and uptake of the vitamin into neurons involves the low-density lipoprotein receptor-related protein 1. Taken together, our data indicate that TTP-expressing astrocytes control the delivery of vitamin E from astrocytes to neurons, and that this process is homeostatically responsive to oxidative stress. These are the first observations that address the detailed molecular mechanisms of vitamin E transport in the central nervous system, and these results have important implications for understanding the molecular underpinnings of oxidative stress-related neurodegenerative diseases.
Keywords: lipid trafficking; lipid transport; tocopherol; tocopherol transfer protein; vitamin E.
Copyright © 2022 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Conflict of interest The authors declare that they have no conflicts of interest with the contents of this article.
Figures

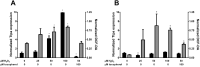
References
-
- Burton G.W., Cheeseman K.H., Doba T., Ingold K.U., Slater T.F. Vitamin E as an antioxidant in vitro and in vivo. Ciba Found. Symp. 1983;101:4–18. - PubMed
-
- Ingold K.U., Webb A.C., Witter D., Burton G.W., Metcalfe T.A., Muller D.P. Vitamin E remains the major lipid-soluble, chain-breaking antioxidant in human plasma even in individuals suffering severe vitamin E deficiency. Arch. Biochem. Biophys. 1987;259:224–225. - PubMed
-
- Muller D.P., Lloyd J.K. Effect of large oral doses of vitamin E on the neurological sequelae of patients with abetalipoproteinemia. Ann. N. Y. Acad. Sci. 1982;393:133–144. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

