Integrin mediates cell entry of the SARS-CoV-2 virus independent of cellular receptor ACE2
- PMID: 35150743
- PMCID: PMC8828381
- DOI: 10.1016/j.jbc.2022.101710
Integrin mediates cell entry of the SARS-CoV-2 virus independent of cellular receptor ACE2
Abstract
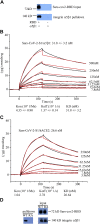
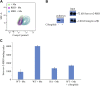
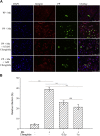
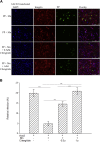
Coronavirus disease 2019 (COVID-19) is a highly contagious disease caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). It is broadly accepted that SARS-CoV-2 utilizes its spike protein to recognize the extracellular domain of angiotensin-converting enzyme 2 (ACE2) to enter cells for viral infection. However, other mechanisms of SARS-CoV-2 cell entry may occur. We show quantitatively that the SARS-CoV-2 spike protein also binds to the extracellular domain of broadly expressed integrin α5β1 with an affinity comparable to that of SARS-CoV-2 binding to ACE2. More importantly, we provide direct evidence that such binding promotes the internalization of SARS-CoV-2 into non-ACE2 cells in a manner critically dependent upon the activation of the integrin. Our data demonstrate an alternative pathway for the cell entry of SARS-CoV-2, suggesting that upon initial ACE2-mediated invasion of the virus in the respiratory system, which is known to trigger an immune response and secretion of cytokines to activate integrin, the integrin-mediated cell invasion of SARS-CoV-2 into the respiratory system and other organs becomes effective, thereby promoting further infection and progression of COVID-19.
Keywords: COVID-19; SARS-CoV-2; integrin; virus infection.
Copyright © 2022 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Conflict of interest The authors declare that they have no conflicts of interest with the contents of this article.
Figures





References
-
- Lan J., Ge J., Yu J., Shan S., Zhou H., Fan S., Zhang Q., Shi X., Wang Q., Zhang L., Wang X. Structure of the SARS-CoV-2 spike receptor-binding domain bound to the ACE2 receptor. Nature. 2020;581:215–220. - PubMed
-
- Donoghue M., Hsieh F., Baronas E., Godbout K., Gosselin M., Stagliano N., Donovan M., Woolf B., Robison K., Jeyaseelan R., Breitbart R.E., Acton S. A novel angiotensin-converting enzyme-related carboxypeptidase (ACE2) converts angiotensin I to angiotensin 1-9. Circ. Res. 2000;87:E1–E9. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

