Skeletal muscle undergoes fiber type metabolic switch without myosin heavy chain switch in response to defective fatty acid oxidation
- PMID: 35150906
- PMCID: PMC8898976
- DOI: 10.1016/j.molmet.2022.101456
Skeletal muscle undergoes fiber type metabolic switch without myosin heavy chain switch in response to defective fatty acid oxidation
Abstract
Objective: Skeletal muscle is a heterogeneous and dynamic tissue that adapts to functional demands and substrate availability by modulating muscle fiber size and type. The concept of muscle fiber type relates to its contractile (slow or fast) and metabolic (glycolytic or oxidative) properties. Here, we tested whether disruptions in muscle oxidative catabolism are sufficient to prompt parallel adaptations in energetics and contractile protein composition.
Methods: Mice with defective mitochondrial long-chain fatty acid oxidation (mLCFAO) in the skeletal muscle due to loss of carnitine palmitoyltransferase 2 (Cpt2Sk-/-) were used to model a shift in muscle macronutrient catabolism. Glycolytic and oxidative muscles of Cpt2Sk-/- mice and control littermates were compared for the expression of energy metabolism-related proteins, mitochondrial respiratory capacity, and myosin heavy chain isoform composition.
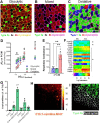
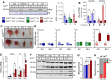
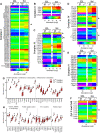
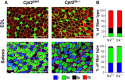
Results: Differences in bioenergetics and macronutrient utilization in response to energy demands between control muscles were intrinsic to the mitochondria, allowing for a clear distinction of muscle types. Loss of CPT2 ablated mLCFAO and resulted in mitochondrial biogenesis occurring most predominantly in oxidative muscle fibers. The metabolism-related proteomic signature of Cpt2Sk-/- oxidative muscle more closely resembled that of glycolytic muscle than of control oxidative muscle. Respectively, intrinsic substrate-supported mitochondrial respiration of CPT2 deficient oxidative muscles shifted to closely match that of glycolytic muscles. Despite this shift in mitochondrial metabolism, CPT2 deletion did not result in contractile-based fiber type switching according to myosin heavy chain composition analysis.
Conclusion: The loss of mitochondrial long-chain fatty acid oxidation elicits an adaptive response involving conversion of oxidative muscle toward a metabolic profile that resembles a glycolytic muscle, but this is not accompanied by changes in myosin heavy chain isoforms. These data suggest that shifts in muscle catabolism are not sufficient to drive shifts in the contractile apparatus but are sufficient to drive adaptive changes in metabolic properties.
Keywords: Bioenergetics; Carnitine palmitoyltransferase 2; Fatty acid oxidation; Fiber-typing; Mitochondrial biogenesis; Skeletal muscle.
Copyright © 2022 The Author(s). Published by Elsevier GmbH.. All rights reserved.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

